Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics
- PMID: 21251258
- PMCID: PMC3032642
- DOI: 10.1186/1471-2180-11-16
Induction kinetics of the Staphylococcus aureus cell wall stress stimulon in response to different cell wall active antibiotics
Abstract
Background: Staphylococcus aureus activates a protective cell wall stress stimulon (CWSS) in response to the inhibition of cell wall synthesis or cell envelope damage caused by several structurally and functionally different antibiotics. CWSS induction is coordinated by the VraSR two-component system, which senses an unknown signal triggered by diverse cell wall active agents.
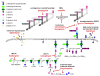
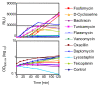
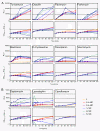
Results: We have constructed a highly sensitive luciferase reporter gene system, using the promoter of sas016 (S. aureus N315), which detects very subtle differences in expression as well as measuring > 4 log-fold changes in CWSS activity, to compare the concentration dependence of CWSS induction kinetics of antibiotics with different cell envelope targets. We compared the effects of subinhibitory up to suprainhibitory concentrations of fosfomycin, D-cycloserine, tunicamycin, bacitracin, flavomycin, vancomycin, teicoplanin, oxacillin, lysostaphin and daptomycin. Induction kinetics were both strongly antibiotic- and concentration-dependent. Most antibiotics triggered an immediate response with induction beginning within 10 min, except for tunicamycin, D-cycloserine and fosfomycin which showed lags of up to one generation before induction commenced. Induction characteristics, such as the rate of CWSS induction once initiated and maximal induction reached, were strongly antibiotic dependent. We observed a clear correlation between the inhibitory effects of specific antibiotic concentrations on growth and corresponding increases in CWSS induction kinetics. Inactivation of VraR increased susceptibility to the antibiotics tested from 2- to 16-fold, with the exceptions of oxacillin and D-cycloserine, where no differences were detected in the methicillin susceptible S. aureus strain background analysed. There was no apparent correlation between the induction capacity of the various antibiotics and the relative importance of the CWSS for the corresponding resistance phenotypes.
Conclusion: CWSS induction profiles were unique for each antibiotic. Differences observed in optimal induction conditions for specific antibiotics should be determined and taken into account when designing and interpreting CWSS induction studies.
Figures


Similar articles
-
Mutational analyses of open reading frames within the vraSR operon and their roles in the cell wall stress response of Staphylococcus aureus.Antimicrob Agents Chemother. 2011 Apr;55(4):1391-402. doi: 10.1128/AAC.01213-10. Epub 2011 Jan 10. Antimicrob Agents Chemother. 2011. PMID: 21220524 Free PMC article.
-
Deletion of hypothetical wall teichoic acid ligases in Staphylococcus aureus activates the cell wall stress response.FEMS Microbiol Lett. 2012 Aug;333(2):109-20. doi: 10.1111/j.1574-6968.2012.02603.x. Epub 2012 Jun 18. FEMS Microbiol Lett. 2012. PMID: 22640011
-
Luciferase Reporter Gene System to Detect Cell Wall Stress Stimulon Induction in Staphylococcus aureus.Methods Mol Biol. 2016;1440:139-50. doi: 10.1007/978-1-4939-3676-2_11. Methods Mol Biol. 2016. PMID: 27311670
-
Transcriptional profiling reveals that daptomycin induces the Staphylococcus aureus cell wall stress stimulon and genes responsive to membrane depolarization.Antimicrob Agents Chemother. 2008 Mar;52(3):980-90. doi: 10.1128/AAC.01121-07. Epub 2007 Dec 17. Antimicrob Agents Chemother. 2008. PMID: 18086846 Free PMC article.
-
Targeting membrane-bound bacterial cell wall precursors: a tried and true antibiotic strategy in nature and the clinic.Chem Commun (Camb). 2023 Jun 20;59(50):7685-7703. doi: 10.1039/d3cc01070h. Chem Commun (Camb). 2023. PMID: 37219335 Review.
Cited by
-
Targeting cell membrane adaptation as a novel antimicrobial strategy.Curr Opin Microbiol. 2016 Oct;33:91-96. doi: 10.1016/j.mib.2016.07.002. Epub 2016 Jul 25. Curr Opin Microbiol. 2016. PMID: 27458841 Free PMC article. Review.
-
Polymyxin and lipopeptide antibiotics: membrane-targeting drugs of last resort.Microbiology (Reading). 2022 Feb;168(2):001136. doi: 10.1099/mic.0.001136. Microbiology (Reading). 2022. PMID: 35118938 Free PMC article. Review.
-
Methionine sulfoxide reductases protect against oxidative stress in Staphylococcus aureus encountering exogenous oxidants and human neutrophils.J Innate Immun. 2014;6(3):353-64. doi: 10.1159/000355915. Epub 2013 Nov 15. J Innate Immun. 2014. PMID: 24247266 Free PMC article.
-
Tea tree oil-induced transcriptional alterations in Staphylococcus aureus.Phytother Res. 2013 Mar;27(3):390-6. doi: 10.1002/ptr.4738. Epub 2012 May 23. Phytother Res. 2013. PMID: 22619070 Free PMC article.
-
Potent small-molecule suppression of oxacillin resistance in methicillin-resistant Staphylococcus aureus.Angew Chem Int Ed Engl. 2012 Nov 5;51(45):11254-7. doi: 10.1002/anie.201206911. Epub 2012 Oct 9. Angew Chem Int Ed Engl. 2012. PMID: 23047322 Free PMC article.
References
-
- Utaida S, Dunman PM, Macapagal D, Murphy E, Projan SJ, Singh VK, Jayaswal RK, Wilkinson BJ. Genome-wide transcriptional profiling of the response of Staphylococcus aureus to cell-wall-active antibiotics reveals a cell-wall-stress stimulon. Microbiology. 2003;149(Pt 10):2719–2732. doi: 10.1099/mic.0.26426-0. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

