An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice
- PMID: 21248316
- PMCID: PMC3138523
- DOI: 10.1126/scitranslmed.3001581
An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice
Abstract
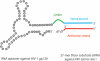
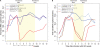
Therapeutic strategies designed to treat HIV infection with combinations of antiviral drugs have proven to be the best approach for slowing the progression to AIDS. Despite this progress, there are problems with viral drug resistance and toxicity, necessitating new approaches to combating HIV-1 infection. We have therefore developed a different combination approach for the treatment of HIV infection in which an RNA aptamer, with high binding affinity to the HIV-1 envelope (gp120) protein and virus neutralization properties, is attached to and delivers a small interfering RNA (siRNA) that triggers sequence-specific degradation of HIV RNAs. We have tested the antiviral activities of these chimeric RNAs in a humanized Rag2(-/-)γc(-/-) (RAG-hu) mouse model with multilineage human hematopoiesis. In this animal model, HIV-1 replication and CD4(+) T cell depletion mimic the situation seen in human HIV-infected patients. Our results show that treatment with either the anti-gp120 aptamer or the aptamer-siRNA chimera suppressed HIV-1 replication by several orders of magnitude and prevented the viral-induced helper CD4(+) T cell decline. In comparison to the aptamer alone, the aptamer-siRNA combination provided more extensive inhibition, resulting in a significantly longer antiviral effect that extended several weeks beyond the last injected dose. The aptamer thus acts as a broad-spectrum HIV-neutralizing agent and an siRNA delivery vehicle. The combined aptamer-siRNA agent provides an attractive, nontoxic therapeutic approach for treatment of HIV infection.
Figures



Similar articles
-
Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1.Theranostics. 2018 Feb 7;8(6):1575-1590. doi: 10.7150/thno.23085. eCollection 2018. Theranostics. 2018. PMID: 29556342 Free PMC article.
-
Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice.Mol Ther. 2011 Dec;19(12):2228-38. doi: 10.1038/mt.2011.207. Epub 2011 Sep 27. Mol Ther. 2011. PMID: 21952167 Free PMC article.
-
Development of cell-type specific anti-HIV gp120 aptamers for siRNA delivery.J Vis Exp. 2011 Jun 23;(52):2954. doi: 10.3791/2954. J Vis Exp. 2011. PMID: 21730942 Free PMC article.
-
Therapeutic potential of aptamer-siRNA conjugates for treatment of HIV-1.BioDrugs. 2012 Dec 1;26(6):393-400. doi: 10.2165/11635350-000000000-00000. BioDrugs. 2012. PMID: 23046156 Free PMC article. Review.
-
Cell-type-specific aptamer and aptamer-small interfering RNA conjugates for targeted human immunodeficiency virus type 1 therapy.J Investig Med. 2014 Oct;62(7):914-9. doi: 10.1097/JIM.0000000000000103. J Investig Med. 2014. PMID: 25118114 Free PMC article. Review.
Cited by
-
Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice.Blood. 2012 Nov 29;120(23):4571-82. doi: 10.1182/blood-2012-04-422303. Epub 2012 Oct 11. Blood. 2012. PMID: 23065154 Free PMC article.
-
Delivery of chemo-sensitizing siRNAs to HER2+-breast cancer cells using RNA aptamers.Nucleic Acids Res. 2012 Jul;40(13):6319-37. doi: 10.1093/nar/gks294. Epub 2012 Mar 30. Nucleic Acids Res. 2012. PMID: 22467215 Free PMC article.
-
Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer.Sci Rep. 2016 Jul 26;6:30346. doi: 10.1038/srep30346. Sci Rep. 2016. PMID: 27456457 Free PMC article.
-
Transcriptional gene silencing in humans.Nucleic Acids Res. 2016 Aug 19;44(14):6505-17. doi: 10.1093/nar/gkw139. Epub 2016 Apr 7. Nucleic Acids Res. 2016. PMID: 27060137 Free PMC article.
-
HIV-1 immunopathogenesis in humanized mouse models.Cell Mol Immunol. 2012 May;9(3):237-44. doi: 10.1038/cmi.2012.7. Epub 2012 Apr 16. Cell Mol Immunol. 2012. PMID: 22504952 Free PMC article. Review.
References
-
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. - PubMed
-
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. - PubMed
-
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. - PubMed
-
- Chu TC, Marks JW, III, Lavery LA, Faulkner S, Rosenblum MG, Ellington AD, Levy M. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 2006;66:5989–5992. - PubMed
-
- Mayer G. The chemical biology of aptamers. Angew. Chem. Int. Ed. Engl. 2009;48:2672–2689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI057066/AI/NIAID NIH HHS/United States
- AI29329/AI/NIAID NIH HHS/United States
- R01 HL074704/HL/NHLBI NIH HHS/United States
- R01 AI057066-05/AI/NIAID NIH HHS/United States
- P30 AI054907/AI/NIAID NIH HHS/United States
- HL07470/HL/NHLBI NIH HHS/United States
- R01 AI042552/AI/NIAID NIH HHS/United States
- R01 AI029329/AI/NIAID NIH HHS/United States
- R01 AI073255-04/AI/NIAID NIH HHS/United States
- R01 AI073255/AI/NIAID NIH HHS/United States
- AI073255/AI/NIAID NIH HHS/United States
- R01 AI057066/AI/NIAID NIH HHS/United States
- R37 AI029329/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

