Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice
- PMID: 21245575
- PMCID: PMC3026737
- DOI: 10.1172/JCI44907
Maternal T cells limit engraftment after in utero hematopoietic cell transplantation in mice
Abstract
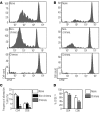
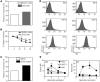
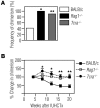
Transplantation of allogeneic stem cells into the early gestational fetus, a treatment termed in utero hematopoietic cell transplantation (IUHCTx), could potentially overcome the limitations of bone marrow transplants, including graft rejection and the chronic immunosuppression required to prevent rejection. However, clinical use of IUHCTx has been hampered by poor engraftment, possibly due to a host immune response against the graft. Since the fetal immune system is relatively immature, we hypothesized that maternal cells trafficking into the fetus may pose the true barrier to effective IUHCTx. Here, we have demonstrated that there is macrochimerism of maternal leukocytes in the blood of unmanipulated mouse fetuses, with substantial increases in T cell trafficking after IUHCTx. To determine the contribution of these maternal lymphocytes to rejection after IUHCTx, we bred T and/or B cell-deficient mothers to wild-type fathers and performed allogeneic IUHCTx into the immunocompetent fetuses. There was a marked improvement in engraftment if the mother lacked T cells but not B cells, indicating that maternal T cells are the main barrier to engraftment. Furthermore, when the graft was matched to the mother, there was no difference in engraftment between syngeneic and allogeneic fetal recipients. Our study suggests that the clinical success of IUHCTx may be improved by transplanting cells matched to the mother.
Figures
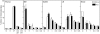
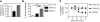


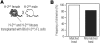
Similar articles
-
Pre-Existing Maternal Antibodies Cause Rapid Prenatal Rejection of Allotransplants in the Mouse Model of In Utero Hematopoietic Cell Transplantation.J Immunol. 2018 Sep 1;201(5):1549-1557. doi: 10.4049/jimmunol.1800183. Epub 2018 Jul 18. J Immunol. 2018. PMID: 30021770 Free PMC article.
-
Multilineage engraftment with minimal graft-versus-host disease following in utero transplantation of S-59 psoralen/ultraviolet a light-treated, sensitized T cells and adult T cell-depleted bone marrow in fetal mice.J Immunol. 2002 Dec 1;169(11):6133-40. doi: 10.4049/jimmunol.169.11.6133. J Immunol. 2002. PMID: 12444116
-
Direct and indirect antigen presentation lead to deletion of donor-specific T cells after in utero hematopoietic cell transplantation in mice.Blood. 2013 May 30;121(22):4595-602. doi: 10.1182/blood-2012-10-463174. Epub 2013 Apr 22. Blood. 2013. PMID: 23610372 Free PMC article.
-
Hematopoietic cell transplantation for the induction of allo- and xenotolerance.Clin Transplant. 1996 Aug;10(4):357-63. Clin Transplant. 1996. PMID: 8884109 Review.
-
In utero hematopoietic cell transplantation for the treatment of congenital anomalies.Clin Perinatol. 2012 Jun;39(2):301-10. doi: 10.1016/j.clp.2012.04.004. Epub 2012 May 8. Clin Perinatol. 2012. PMID: 22682381 Review.
Cited by
-
Prenatal Somatic Cell Gene Therapies: Charting a Path Toward Clinical Applications (Proceedings of the CERSI-FDA Meeting).J Clin Pharmacol. 2022 Sep;62 Suppl 1(Suppl 1):S36-S52. doi: 10.1002/jcph.2127. J Clin Pharmacol. 2022. PMID: 36106778 Free PMC article. Review.
-
In utero therapy for congenital disorders using amniotic fluid stem cells.Front Pharmacol. 2014 Dec 19;5:270. doi: 10.3389/fphar.2014.00270. eCollection 2014. Front Pharmacol. 2014. PMID: 25566071 Free PMC article.
-
Depletion of murine fetal hematopoietic stem cells with c-Kit receptor and CD47 blockade improves neonatal engraftment.Blood Adv. 2018 Dec 26;2(24):3602-3607. doi: 10.1182/bloodadvances.2018022020. Blood Adv. 2018. PMID: 30567724 Free PMC article.
-
Partial rescue of mucopolysaccharidosis type VII mice with a lifelong engraftment of allogeneic stem cells in utero.Congenit Anom (Kyoto). 2015 Feb;55(1):55-64. doi: 10.1111/cga.12099. Congenit Anom (Kyoto). 2015. PMID: 25421592 Free PMC article.
-
Increased maternal T cell microchimerism in the allogeneic fetus during LPS-induced preterm labor in mice.Chimerism. 2014;5(3-4):68-74. doi: 10.1080/19381956.2014.1002703. Epub 2015 Mar 16. Chimerism. 2014. PMID: 25779065 Free PMC article.
References
-
- Hayashi S, Peranteau WH, Shaaban AF, Flake AW. Complete allogeneic hematopoietic chimerism achieved by a combined strategy of in utero hematopoietic stem cell transplantation and postnatal donor lymphocyte infusion. Blood. 2002;100(3):804–812. - PubMed

