In vitro characterization of GS-8374, a novel phosphonate-containing inhibitor of HIV-1 protease with a favorable resistance profile
- PMID: 21245449
- PMCID: PMC3067135
- DOI: 10.1128/AAC.01183-10
In vitro characterization of GS-8374, a novel phosphonate-containing inhibitor of HIV-1 protease with a favorable resistance profile
Abstract
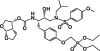
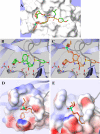
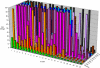
GS-8374 is a novel bis-tetrahydrofuran HIV-1 protease (PR) inhibitor (PI) with a unique diethylphosphonate moiety. It was selected from a series of analogs containing various di(alkyl)phosphonate substitutions connected via a linker to the para position of a P-1 phenyl ring. GS-8374 inhibits HIV-1 PR with high potency (K(i) = 8.1 pM) and with no known effect on host proteases. Kinetic and thermodynamic analysis of GS-8374 binding to PR demonstrated an extremely slow off rate for the inhibitor and favorable contributions of both the enthalpic and entropic components to the total free binding energy. GS-8374 showed potent antiretroviral activity in T-cell lines, primary CD4(+) T cells (50% effective concentration [EC(50)] = 3.4 to 11.5 nM), and macrophages (EC(50) = 25.5 nM) and exhibited low cytotoxicity in multiple human cell types. The antiviral potency of GS-8374 was only moderately affected by human serum protein binding, and its combination with multiple approved antiretrovirals showed synergistic effects. When it was tested in a PhenoSense assay against a panel of 24 patient-derived viruses with high-level PI resistance, GS-8374 showed lower mean EC(50)s and lower fold resistance than any of the clinically approved PIs. Similar to other PIs, in vitro hepatic microsomal metabolism of GS-8374 was efficiently blocked by ritonavir, suggesting a potential for effective pharmacokinetic boosting in vivo. In summary, results from this broad in vitro pharmacological profiling indicate that GS-8374 is a promising candidate to be further assessed as a new antiretroviral agent with potential for clinical efficacy in both treatment-naïve and -experienced patients.
Figures
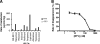
Similar articles
-
GS-8374, a prototype phosphonate-containing inhibitor of HIV-1 protease, effectively inhibits protease mutants with amino acid insertions.J Virol. 2014 Mar;88(6):3586-90. doi: 10.1128/JVI.02688-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371077 Free PMC article.
-
Novel Central Nervous System (CNS)-Targeting Protease Inhibitors for Drug-Resistant HIV Infection and HIV-Associated CNS Complications.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00466-19. doi: 10.1128/AAC.00466-19. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31061155 Free PMC article.
-
Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro.Antimicrob Agents Chemother. 2003 Oct;47(10):3123-9. doi: 10.1128/AAC.47.10.3123-3129.2003. Antimicrob Agents Chemother. 2003. PMID: 14506019 Free PMC article.
-
Darunavir: a review of its use in the management of HIV infection in adults.Drugs. 2009;69(4):477-503. doi: 10.2165/00003495-200969040-00007. Drugs. 2009. PMID: 19323590 Review.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
Cited by
-
Preclinical characterization of a non-peptidomimetic HIV protease inhibitor with improved metabolic stability.Antimicrob Agents Chemother. 2024 Apr 3;68(4):e0137323. doi: 10.1128/aac.01373-23. Epub 2024 Feb 21. Antimicrob Agents Chemother. 2024. PMID: 38380945 Free PMC article.
-
Enhancing protein backbone binding--a fruitful concept for combating drug-resistant HIV.Angew Chem Int Ed Engl. 2012 Feb 20;51(8):1778-802. doi: 10.1002/anie.201102762. Epub 2012 Jan 31. Angew Chem Int Ed Engl. 2012. PMID: 22290878 Free PMC article. Review.
-
GS-8374, a novel HIV protease inhibitor, does not alter glucose homeostasis in cultured adipocytes or in a healthy-rodent model system.Antimicrob Agents Chemother. 2011 Apr;55(4):1377-82. doi: 10.1128/AAC.01184-10. Epub 2011 Jan 18. Antimicrob Agents Chemother. 2011. PMID: 21245443 Free PMC article.
-
Inhibiting HTLV-1 Protease: A Viable Antiviral Target.ACS Chem Biol. 2021 Mar 19;16(3):529-538. doi: 10.1021/acschembio.0c00975. Epub 2021 Feb 23. ACS Chem Biol. 2021. PMID: 33619959 Free PMC article.
-
HIV-1 protease inhibitors with a P1 phosphonate modification maintain potency against drug-resistant variants by increased interactions with flap residues.Eur J Med Chem. 2023 Sep 5;257:115501. doi: 10.1016/j.ejmech.2023.115501. Epub 2023 May 18. Eur J Med Chem. 2023. PMID: 37244161 Free PMC article.
References
-
- Bartlett, J. A., R. DeMasi, J. Quinn, C. Moxham, and F. Rousseau. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS 15:1369-1377. - PubMed
-
- Callebaut, C., et al. 2007. In vitro HIV-1 resistance selection to GS-8374, a novel phosphonate protease inhibitor: comparison with lopinavir, atazanavir, and darunavir, abstr. 16. Abstr. 16th Int. HIV Drug Resistance Workshop.
-
- Carr, A. 2003. Toxicity of antiretroviral therapy and implications for drug development. Nat. Rev. Drug Discov. 2:624-634. - PubMed
-
- Cihlar, T., et al. 2006. Suppression of HIV-1 protease inhibitor resistance by phosphonate-mediated solvent anchoring. J. Mol. Biol. 363:635-647. - PubMed
-
- Clotet, B., et al. 2007. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet 369:1169-1178. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

