miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells
- PMID: 21245307
- PMCID: PMC3038748
- DOI: 10.1073/pnas.1016646108
miR-96 regulates the progression of differentiation in mammalian cochlear inner and outer hair cells
Abstract
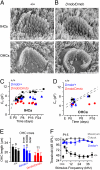
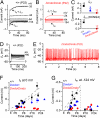
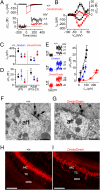
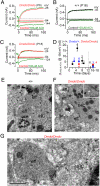
MicroRNAs (miRNAs) are small noncoding RNAs able to regulate a broad range of protein-coding genes involved in many biological processes. miR-96 is a sensory organ-specific miRNA expressed in the mammalian cochlea during development. Mutations in miR-96 cause nonsyndromic progressive hearing loss in humans and mice. The mouse mutant diminuendo has a single base change in the seed region of the Mir96 gene leading to widespread changes in the expression of many genes. We have used this mutant to explore the role of miR-96 in the maturation of the auditory organ. We found that the physiological development of mutant sensory hair cells is arrested at around the day of birth, before their biophysical differentiation into inner and outer hair cells. Moreover, maturation of the hair cell stereocilia bundle and remodelling of auditory nerve connections within the cochlea fail to occur in miR-96 mutants. We conclude that miR-96 regulates the progression of the physiological and morphological differentiation of cochlear hair cells and, as such, coordinates one of the most distinctive functional refinements of the mammalian auditory system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Gata3 is required for the functional maturation of inner hair cells and their innervation in the mouse cochlea.J Physiol. 2019 Jul;597(13):3389-3406. doi: 10.1113/JP277997. Epub 2019 May 28. J Physiol. 2019. PMID: 31069810 Free PMC article.
-
A mouse model of miR-96, miR-182 and miR-183 misexpression implicates miRNAs in cochlear cell fate and homeostasis.Sci Rep. 2018 Feb 23;8(1):3569. doi: 10.1038/s41598-018-21811-1. Sci Rep. 2018. PMID: 29476110 Free PMC article.
-
Localization of efferent neurotransmitters in the inner ear of the homozygous Bronx waltzer mutant mouse.Hear Res. 2002 May;167(1-2):136-55. doi: 10.1016/s0378-5955(02)00382-9. Hear Res. 2002. PMID: 12117537
-
Role of microRNAs in inner ear development and hearing loss.Gene. 2019 Feb 20;686:49-55. doi: 10.1016/j.gene.2018.10.075. Epub 2018 Oct 31. Gene. 2019. PMID: 30389561 Free PMC article. Review.
-
Kölliker's organ-supporting cells and cochlear auditory development.Front Mol Neurosci. 2022 Oct 11;15:1031989. doi: 10.3389/fnmol.2022.1031989. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36304996 Free PMC article. Review.
Cited by
-
The microRNA-183 cluster: the family that plays together stays together.Nucleic Acids Res. 2015 Sep 3;43(15):7173-88. doi: 10.1093/nar/gkv703. Epub 2015 Jul 13. Nucleic Acids Res. 2015. PMID: 26170234 Free PMC article. Review.
-
The long and short: Non-coding RNAs in the mammalian inner ear.Hear Res. 2023 Feb;428:108666. doi: 10.1016/j.heares.2022.108666. Epub 2022 Dec 16. Hear Res. 2023. PMID: 36566643 Free PMC article. Review.
-
A critical period of prehearing spontaneous Ca2+ spiking is required for hair-bundle maintenance in inner hair cells.EMBO J. 2023 Feb 15;42(4):e112118. doi: 10.15252/embj.2022112118. Epub 2023 Jan 3. EMBO J. 2023. PMID: 36594367 Free PMC article.
-
Fasudil prevents neomycin-induced hair cell damage by inhibiting autophagy through the miR-489/NDP52 signaling pathway in HEI-OC1 cells.Exp Ther Med. 2022 Jan;23(1):43. doi: 10.3892/etm.2021.10965. Epub 2021 Nov 12. Exp Ther Med. 2022. PMID: 34849158 Free PMC article.
-
NPTX2 and cognitive dysfunction in Alzheimer's Disease.Elife. 2017 Mar 23;6:e23798. doi: 10.7554/eLife.23798. Elife. 2017. PMID: 28440221 Free PMC article.
References
-
- Liberman MC, et al. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. - PubMed
-
- Guinan JJ., Jr. Physiology of olivocochlear efferents. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 435–502.
-
- Dror AA, Avraham KB. Hearing loss: Mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

