Catalysis and mechanistic insights into sirtuin activation
- PMID: 21243715
- PMCID: PMC3327882
- DOI: 10.1002/cbic.201000434
Catalysis and mechanistic insights into sirtuin activation
Abstract
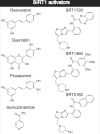
SIRT1 is a member of the Sir2 family of NAD(+)-dependent protein deacetylases. The central role of SIRT1 in multiple metabolic and age-related pathways has pushed SIRT1 to the forefront to discover small-molecule activators. Promising compounds, including resveratrol and SRT1720 have been reported, however, whether these compounds are direct activators and the mechanism by which they activate remains poorly defined. This review examines the current debate surrounding purported activators, and will focus on the assays used in screening compounds, sirtuin catalysis, and the mechanistic basis for their actions. We discuss the potential pathways of SIRT1 activation that could be exploited for the development of novel therapeutics for treating type II diabetes, neurodegeneration, and diseases associated with aging.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
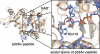
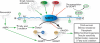
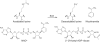


Similar articles
-
SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1.J Biol Chem. 2010 Mar 12;285(11):8340-51. doi: 10.1074/jbc.M109.088682. Epub 2010 Jan 8. J Biol Chem. 2010. PMID: 20061378 Free PMC article.
-
Sirtuin 1 (SIRT1): the misunderstood HDAC.J Biomol Screen. 2011 Dec;16(10):1153-69. doi: 10.1177/1087057111422103. Epub 2011 Nov 15. J Biomol Screen. 2011. PMID: 22086720 Review.
-
Small molecule activators of SIRT1 replicate signaling pathways triggered by calorie restriction in vivo.BMC Syst Biol. 2009 Mar 10;3:31. doi: 10.1186/1752-0509-3-31. BMC Syst Biol. 2009. PMID: 19284563 Free PMC article.
-
A patent review of sirtuin activators: an update.Expert Opin Ther Pat. 2012 Apr;22(4):355-67. doi: 10.1517/13543776.2012.669374. Epub 2012 Mar 9. Expert Opin Ther Pat. 2012. PMID: 22475539 Review.
-
Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol.Genes Dev. 2015 Jun 15;29(12):1316-25. doi: 10.1101/gad.265462.115. Genes Dev. 2015. PMID: 26109052 Free PMC article.
Cited by
-
Obesity and Male Reproduction: Do Sirtuins Play a Role?Int J Mol Sci. 2022 Jan 16;23(2):973. doi: 10.3390/ijms23020973. Int J Mol Sci. 2022. PMID: 35055159 Free PMC article. Review.
-
Sirtuin Deacetylation Mechanism and Catalytic Role of the Dynamic Cofactor Binding Loop.J Phys Chem Lett. 2013 Feb 7;4(3):491-495. doi: 10.1021/jz302015s. J Phys Chem Lett. 2013. PMID: 23585919 Free PMC article.
-
Epigenetic pathway targets for the treatment of disease: accelerating progress in the development of pharmacological tools: IUPHAR Review 11.Br J Pharmacol. 2014 Nov;171(22):4981-5010. doi: 10.1111/bph.12848. Br J Pharmacol. 2014. PMID: 25060293 Free PMC article. Review.
-
Sirtuin catalysis and regulation.J Biol Chem. 2012 Dec 14;287(51):42419-27. doi: 10.1074/jbc.R112.378877. Epub 2012 Oct 18. J Biol Chem. 2012. PMID: 23086947 Free PMC article. Review.
-
The role of sirtuins in cellular homeostasis.J Physiol Biochem. 2016 Sep;72(3):371-80. doi: 10.1007/s13105-016-0492-6. Epub 2016 May 6. J Physiol Biochem. 2016. PMID: 27154583 Free PMC article. Review.
References
-
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Nature. 2007;450:712. - PMC - PubMed
- Milne J, Denu J. Curr. Opin. Chem. Biol. 2008;12:11. - PubMed
-
- Gasser S, Cockell M. Gene. 2001;279:1. - PubMed
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Nature. 2003;425:191. - PubMed
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Nature. 2004;430:686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

