The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice
- PMID: 21239643
- PMCID: PMC3051255
- DOI: 10.1105/tpc.110.080325
The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice
Abstract
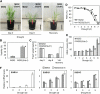
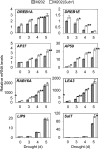
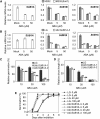
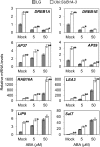
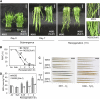
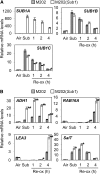
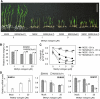
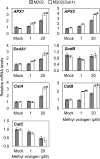
Submergence and drought are major constraints to rice (Oryza sativa) production in rain-fed farmlands, both of which can occur sequentially during a single crop cycle. SUB1A, an ERF transcription factor found in limited rice accessions, dampens ethylene production and gibberellic acid responsiveness during submergence, economizing carbohydrate reserves and significantly prolonging endurance. Here, we evaluated the functional role of SUB1A in acclimation to dehydration. Comparative analysis of genotypes with and without SUB1A revealed that SUB1A enhanced recovery from drought at the vegetative stage through reduction of leaf water loss and lipid peroxidation and increased expression of genes associated with acclimation to dehydration. Overexpression of SUB1A augmented ABA responsiveness, thereby activating stress-inducible gene expression. Paradoxically, vegetative tissue undergoes dehydration upon desubmergence even though the soil contains sufficient water, indicating that leaf desiccation occurs in the natural progression of a flooding event. Desubmergence caused the upregulation of gene transcripts associated with acclimation to dehydration, with higher induction in SUB1A genotypes. SUB1A also restrained accumulation of reactive oxygen species (ROS) in aerial tissue during drought and desubmergence. Consistently, SUB1A increased the abundance of transcripts encoding ROS scavenging enzymes, resulting in enhanced tolerance to oxidative stress. Therefore, in addition to providing robust submergence tolerance, SUB1A improves survival of rapid dehydration following desubmergence and water deficit during drought.
Figures
Similar articles
-
The key regulator of submergence tolerance, SUB1A, promotes photosynthetic and metabolic recovery from submergence damage in rice leaves.Plant Cell Environ. 2016 Mar;39(3):672-84. doi: 10.1111/pce.12661. Epub 2015 Dec 11. Plant Cell Environ. 2016. PMID: 26477688
-
The submergence tolerance gene SUB1A delays leaf senescence under prolonged darkness through hormonal regulation in rice.Plant Physiol. 2012 Dec;160(4):1795-807. doi: 10.1104/pp.112.207738. Epub 2012 Oct 16. Plant Physiol. 2012. PMID: 23073696 Free PMC article.
-
The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors.Plant Physiol. 2010 Mar;152(3):1674-92. doi: 10.1104/pp.109.152157. Epub 2010 Jan 27. Plant Physiol. 2010. PMID: 20107022 Free PMC article.
-
Unraveling the genetic enigma of rice submergence tolerance: Shedding light on the role of ethylene response factor-encoding gene SUB1A-1.Plant Physiol Biochem. 2024 Jan;206:108224. doi: 10.1016/j.plaphy.2023.108224. Epub 2023 Nov 25. Plant Physiol Biochem. 2024. PMID: 38091930 Review.
-
After The Deluge: Plant Revival Post-Flooding.Trends Plant Sci. 2019 May;24(5):443-454. doi: 10.1016/j.tplants.2019.02.007. Epub 2019 Mar 9. Trends Plant Sci. 2019. PMID: 30857921 Review.
Cited by
-
Reactive oxygen species mediate growth and death in submerged plants.Front Plant Sci. 2013 Jun 4;4:179. doi: 10.3389/fpls.2013.00179. eCollection 2013. Front Plant Sci. 2013. PMID: 23761805 Free PMC article.
-
The ethylene-responsive transcription factor of durum wheat, TdSHN1, confers cadmium, copper, and zinc tolerance to yeast and transgenic tobacco plants.Protoplasma. 2022 Jan;259(1):19-31. doi: 10.1007/s00709-021-01635-z. Epub 2021 Mar 23. Protoplasma. 2022. PMID: 33759027
-
A Positive Feedback Loop Governed by SUB1A1 Interaction with MITOGEN-ACTIVATED PROTEIN KINASE3 Imparts Submergence Tolerance in Rice.Plant Cell. 2016 May;28(5):1127-43. doi: 10.1105/tpc.15.01001. Epub 2016 Apr 14. Plant Cell. 2016. PMID: 27081183 Free PMC article.
-
Ethylene Differentially Modulates Hypoxia Responses and Tolerance across Solanum Species.Plants (Basel). 2020 Aug 13;9(8):1022. doi: 10.3390/plants9081022. Plants (Basel). 2020. PMID: 32823611 Free PMC article.
-
Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance.Plant Physiol. 2011 Aug;156(4):1706-24. doi: 10.1104/pp.111.175570. Epub 2011 May 27. Plant Physiol. 2011. PMID: 21622811 Free PMC article.
References
-
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 - PubMed
-
- Achard P., Renou J.-P., Berthomé R., Harberd N.P., Genschik P. (2008). Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18: 656–660 - PubMed
-
- Acharya B.R., Assmann S.M. (2009). Hormone interactions in stomatal function. Plant Mol. Biol. 69: 451–462 - PubMed
-
- Amir Hossain M., Lee Y., Cho J.-I., Ahn C.H., Lee S.K., Jeon J.S., Kang H., Lee C.H., An G., Park P.B. (2010). The bZIP transcription factor OsABF1 is an ABA responsive element binding factor that enhances abiotic stress signaling in rice. Plant Mol. Biol. 72: 557–566 - PubMed
-
- Badawi G.H., Kawano N., Yamauchi Y., Shimada E., Sasaki R., Kubo A., Tanaka K. (2004). Over-expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol. Plant. 121: 231–238 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

