Up-regulation of MUC18 in airway epithelial cells by IL-13: implications in bacterial adherence
- PMID: 21239604
- PMCID: PMC3095981
- DOI: 10.1165/rcmb.2010-0384OC
Up-regulation of MUC18 in airway epithelial cells by IL-13: implications in bacterial adherence
Abstract
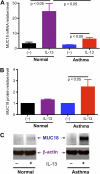
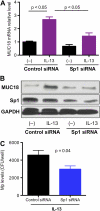
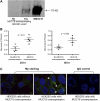
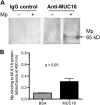
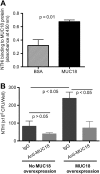
Airway bacterial infections are a major problem in lung diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis. Increased Th2 cytokines, such as IL-13, are observed in lung diseases and may contribute to bacterial infections. How Th2 cytokines affect bacterial infection remains unknown. MUC18, an adhesion molecule shown to be involved in the pathogenesis of malignant melanoma, has been recently identified in airway epithelial cells of patients with COPD. We investigated MUC18 regulation by IL-13 and the role of MUC18 in bacterial adherence to epithelial cells. Human airway tissues, brushed bronchial epithelial cells from normal subjects and subjects with asthma, and epithelial cell lines (e.g., HEK293 cells) were used to study the regulation of MUC18 by IL-13 and the involvement of MUC18 in bacterial (e.g., Mycoplasma pneumoniae [Mp] and nontypeable Haemophilus influenzae [NTHi]) adherence to epithelial cells. Asthmatic bronchial epithelium expressed higher levels of MUC18 than normal bronchial epithelium. IL-13 increased MUC18 in cultured bronchial epithelial cells from normal subjects and particularly from subjects with asthma. IL-13-induced MUC18 expression may be modulated in part through transcription factor specificity protein 1. Overexpression of human MUC18 in HEK293 cells increased cell-associated Mp and NTHi levels. Moreover, MUC18 was shown to directly interact with Mp and NTHi. These results for the first time show that an allergic airway milieu (e.g., IL-13) increases MUC18 expression, which may contribute to increased bacterial infection/colonization in asthma and other lung diseases.
Figures

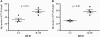
Similar articles
-
A novel function of MUC18: amplification of lung inflammation during bacterial infection.Am J Pathol. 2013 Mar;182(3):819-27. doi: 10.1016/j.ajpath.2012.11.005. Epub 2012 Dec 17. Am J Pathol. 2013. PMID: 23256918 Free PMC article.
-
beta2-agonists promote host defense against bacterial infection in primary human bronchial epithelial cells.BMC Pulm Med. 2010 May 14;10:30. doi: 10.1186/1471-2466-10-30. BMC Pulm Med. 2010. PMID: 20470412 Free PMC article.
-
An antagonist of the platelet-activating factor receptor inhibits adherence of both nontypeable Haemophilus influenzae and Streptococcus pneumoniae to cultured human bronchial epithelial cells exposed to cigarette smoke.Int J Chron Obstruct Pulmon Dis. 2016 Jul 25;11:1647-55. doi: 10.2147/COPD.S108698. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 27524890 Free PMC article.
-
Non-typeable Haemophilus influenzae airways infection: the next treatable trait in asthma?Eur Respir Rev. 2022 Sep 20;31(165):220008. doi: 10.1183/16000617.0008-2022. Print 2022 Sep 30. Eur Respir Rev. 2022. PMID: 36130784 Free PMC article. Review.
-
Bacterial-induced release of inflammatory mediators by bronchial epithelial cells.Eur Respir J. 1996 Sep;9(9):1913-22. doi: 10.1183/09031936.96.09091913. Eur Respir J. 1996. PMID: 8880112 Review.
Cited by
-
CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18.Gene Ther. 2015 Oct;22(10):822-9. doi: 10.1038/gt.2015.53. Epub 2015 Jul 2. Gene Ther. 2015. PMID: 26043872 Free PMC article.
-
A novel function of MUC18: amplification of lung inflammation during bacterial infection.Am J Pathol. 2013 Mar;182(3):819-27. doi: 10.1016/j.ajpath.2012.11.005. Epub 2012 Dec 17. Am J Pathol. 2013. PMID: 23256918 Free PMC article.
-
Knockdown of CD146 promotes endothelial-to-mesenchymal transition via Wnt/β-catenin pathway.PLoS One. 2022 Aug 24;17(8):e0273542. doi: 10.1371/journal.pone.0273542. eCollection 2022. PLoS One. 2022. PMID: 36001597 Free PMC article.
-
Role of epithelial mucins during airway infection.Pulm Pharmacol Ther. 2012 Dec;25(6):415-9. doi: 10.1016/j.pupt.2011.12.003. Epub 2011 Dec 17. Pulm Pharmacol Ther. 2012. PMID: 22198062 Free PMC article. Review.
-
CD146 as a promising therapeutic target for retinal and choroidal neovascularization diseases.Sci China Life Sci. 2022 Jun;65(6):1157-1170. doi: 10.1007/s11427-021-2020-0. Epub 2021 Oct 29. Sci China Life Sci. 2022. PMID: 34729700
References
-
- Jean D, Gershenwald JE, Huang S, Luca M, Hudson MJ, Tainsky MA, Bar-Eli M. Loss of AP-2 results in up-regulation of MCAM/MUC18 and an increase in tumor growth and metastasis of human melanoma cells. J Biol Chem 1998;273:16501–16508. - PubMed
-
- Boneberg EM, Illges H, Legler DF, Furstenberger G. Soluble CD146 is generated by ectodomain shedding of membrane CD146 in a calcium-induced, matrix metalloprotease-dependent process. Microvasc Res 2009;78:325–331. - PubMed
-
- Xie S, Luca M, Huang S, Gutman M, Reich R, Johnson JP, Bar-Eli M. Expression of MCAM/MUC18 by human melanoma cells leads to increased tumor growth and metastasis. Cancer Res 1997;57:2295–2303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

