A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen
- PMID: 21239503
- PMCID: PMC3048660
- DOI: 10.1074/jbc.C110.195768
A novel 3-hydroxyproline (3Hyp)-rich motif marks the triple-helical C terminus of tendon type I collagen
Abstract
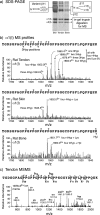
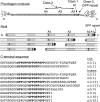
Because of its unique physical and chemical properties, rat tail tendon collagen has long been favored for crystallographic and biochemical studies of fibril structure. In studies of the distribution of 3-hydroxyproline in type I collagen of rat bone, skin, and tail tendon by mass spectrometry, the repeating sequences of Gly-Pro-Pro (GPP) triplets at the C terminus of α1(I) and α2(I) chains were shown to be heavily 3-hydroxylated in tendon but not in skin and bone. By isolating the tryptic peptides and subjecting them to Edman sequence analysis, the presence of repeating 3-hydroxyprolines in consecutive GPP triplets adjacent to 4-hydroxyproline was confirmed as a unique feature of the tendon collagen. A 1960s study by Piez et al. (Piez, K. A., Eigner, E. A., and Lewis, M. S. (1963) Biochemistry 2, 58-66) in which they compared the amino acid compositions of rat skin and tail tendon type I collagen chains indeed showed 3-4 residues of 3Hyp in tendon α1(I) and α2(I) chains but only one 3Hyp residue in skin α1(I) and none in α2(I). The present work therefore confirms this difference and localizes the additional 3Hyp to the GPP repeat at the C terminus of the triple-helix. We speculate on the significance in terms of a potential function in contributing to the unique assembly mechanism and molecular packing in tendon collagen fibrils and on mechanisms that could regulate 3-hydroxylation at this novel substrate site in a tissue-specific manner.
Figures

Similar articles
-
Evolutionary origins of C-terminal (GPP)n 3-hydroxyproline formation in vertebrate tendon collagen.PLoS One. 2014 Apr 2;9(4):e93467. doi: 10.1371/journal.pone.0093467. eCollection 2014. PLoS One. 2014. PMID: 24695516 Free PMC article.
-
Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly.J Biol Chem. 2010 Jan 22;285(4):2580-90. doi: 10.1074/jbc.M109.068726. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940144 Free PMC article.
-
Developmental Stage-dependent Regulation of Prolyl 3-Hydroxylation in Tendon Type I Collagen.J Biol Chem. 2016 Jan 8;291(2):837-47. doi: 10.1074/jbc.M115.686105. Epub 2015 Nov 13. J Biol Chem. 2016. PMID: 26567337 Free PMC article.
-
Structural aspects of hydroxyproline-containing proteins.J Biomol Struct Dyn. 1983 Dec;1(3):843-55. doi: 10.1080/07391102.1983.10507485. J Biomol Struct Dyn. 1983. PMID: 6401122 Review.
-
Role of prolyl hydroxylation in the molecular interactions of collagens.Essays Biochem. 2019 Sep 13;63(3):325-335. doi: 10.1042/EBC20180053. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31350381 Free PMC article. Review.
Cited by
-
Solid-state NMR study reveals collagen I structural modifications of amino acid side chains upon fibrillogenesis.J Biol Chem. 2013 Mar 15;288(11):7528-7535. doi: 10.1074/jbc.M112.390146. Epub 2013 Jan 22. J Biol Chem. 2013. PMID: 23341452 Free PMC article.
-
Conformational landscape of substituted prolines.Biophys Rev. 2020 Feb;12(1):25-39. doi: 10.1007/s12551-020-00621-8. Epub 2020 Jan 17. Biophys Rev. 2020. PMID: 31953795 Free PMC article. Review.
-
Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice.J Biol Chem. 2013 Aug 23;288(34):24742-52. doi: 10.1074/jbc.M113.464156. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861401 Free PMC article.
-
A role for prolyl 3-hydroxylase 2 in post-translational modification of fibril-forming collagens.J Biol Chem. 2011 Sep 2;286(35):30662-30669. doi: 10.1074/jbc.M111.267906. Epub 2011 Jul 11. J Biol Chem. 2011. PMID: 21757687 Free PMC article.
-
Evolutionary origins of C-terminal (GPP)n 3-hydroxyproline formation in vertebrate tendon collagen.PLoS One. 2014 Apr 2;9(4):e93467. doi: 10.1371/journal.pone.0093467. eCollection 2014. PLoS One. 2014. PMID: 24695516 Free PMC article.
References
-
- Ogle J. D., Arlinghaus R. B., Logan M. A. (1962) J. Biol. Chem. 237, 3667–3673 - PubMed
-
- Morello R., Bertin T. K., Chen Y., Hicks J., Tonachini L., Monticone M., Castagnola P., Rauch F., Glorieux F. H., Vranka J., Bächinger H. P., Pace J. M., Schwarze U., Byers P. H., Weis M., Fernandes R. J., Eyre D. R., Yao Z., Boyce B. F., Lee B. (2006) Cell 127, 291–304 - PubMed
-
- Baldridge D., Schwarze U., Morello R., Lennington J., Bertin T. K., Pace J. M., Pepin M. G., Weis M., Eyre D. R., Walsh J., Lambert D., Green A., Robinson H., Michelson M., Houge G., Lindman C., Martin J., Ward J., Lemyre E., Mitchell J. J., Krakow D., Rimoin D. L., Cohn D. H., Byers P. H., Lee B. (2008) Hum. Mutat. 29, 1435–1442 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

