Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats
- PMID: 21232546
- PMCID: PMC3133531
- DOI: 10.1016/j.neuropharm.2010.12.032
Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats
Abstract
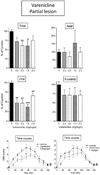
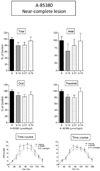
L-dopa therapy for Parkinson's disease leads to dyskinesias or abnormal involuntary movement (AIMs) for which there are few treatment options. Our previous data showed that nicotine administration reduced L-dopa-induced AIMs in parkinsonian monkeys and rats. To further understand how nicotine mediates its antidyskinetic action, we investigated the effect of nicotinic receptor (nAChR) agonists in unilateral 6-OHDA-lesioned rats with varying striatal damage. We first tested the drugs in L-dopa-treated rats with a near-complete striatal dopamine lesion (>99%), the standard rodent dyskinesia model. Varenicline, an agonist that interacts with multiple nAChRs, did not significantly reduce L-dopa-induced AIMs, while 5-iodo-A-85380 (A-85380), which acts selectively at α4β2* and α6β2* subtypes, reduced AIMs by 20%. By contrast, both varenicline and A-85380 reduced L-dopa-induced AIMs by 40-50% in rats with a partial striatal dopamine lesion. Neither drug worsened the antiparkinsonian action of L-dopa. The results show that selective nicotinic agonists reduce dyskinesias, and that they are optimally effective in animals with partial striatal dopamine damage. These findings suggest that presynaptic dopamine terminal α4β2* and α6β2* nAChRs are critical for nicotine's antidyskinetic action. The current data have important implications for the use of nicotinic receptor-directed drugs for L-dopa-induced dyskinesias, a debilitating motor complication of dopamine replacement therapy for Parkinson's disease.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
α4β2 Nicotinic receptors play a role in the nAChR-mediated decline in L-dopa-induced dyskinesias in parkinsonian rats.Neuropharmacology. 2013 Aug;71:191-203. doi: 10.1016/j.neuropharm.2013.03.038. Epub 2013 Apr 12. Neuropharmacology. 2013. PMID: 23583932 Free PMC article.
-
Nicotine reduces L-DOPA-induced dyskinesias by acting at beta2* nicotinic receptors.J Pharmacol Exp Ther. 2011 Sep;338(3):932-41. doi: 10.1124/jpet.111.182949. Epub 2011 Jun 10. J Pharmacol Exp Ther. 2011. PMID: 21665941 Free PMC article.
-
Nicotinic receptor-mediated reduction in L-DOPA-induced dyskinesias may occur via desensitization.J Pharmacol Exp Ther. 2010 Jun;333(3):929-38. doi: 10.1124/jpet.109.162396. Epub 2010 Mar 3. J Pharmacol Exp Ther. 2010. PMID: 20200117 Free PMC article.
-
Role for the nicotinic cholinergic system in movement disorders; therapeutic implications.Pharmacol Ther. 2014 Oct;144(1):50-9. doi: 10.1016/j.pharmthera.2014.05.004. Epub 2014 May 14. Pharmacol Ther. 2014. PMID: 24836728 Free PMC article. Review.
-
Multiple roles for nicotine in Parkinson's disease.Biochem Pharmacol. 2009 Oct 1;78(7):677-85. doi: 10.1016/j.bcp.2009.05.003. Epub 2009 May 9. Biochem Pharmacol. 2009. PMID: 19433069 Free PMC article. Review.
Cited by
-
Neuronal nicotinic receptor agonists improve gait and balance in olivocerebellar ataxia.Neuropharmacology. 2013 Oct;73:75-86. doi: 10.1016/j.neuropharm.2013.05.016. Epub 2013 May 24. Neuropharmacology. 2013. PMID: 23711550 Free PMC article.
-
Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias: studies with parkinsonian nicotinic receptor knockout mice.Biochem Pharmacol. 2013 Oct 15;86(8):1153-62. doi: 10.1016/j.bcp.2013.06.027. Epub 2013 Jul 4. Biochem Pharmacol. 2013. PMID: 23831952 Free PMC article.
-
Evidence for a role for α6(∗) nAChRs in l-dopa-induced dyskinesias using Parkinsonian α6(∗) nAChR gain-of-function mice.Neuroscience. 2015 Jun 4;295:187-97. doi: 10.1016/j.neuroscience.2015.03.040. Epub 2015 Mar 24. Neuroscience. 2015. PMID: 25813704 Free PMC article.
-
Dystonia and levodopa-induced dyskinesias in Parkinson's disease: Is there a connection?Neurobiol Dis. 2019 Dec;132:104579. doi: 10.1016/j.nbd.2019.104579. Epub 2019 Aug 22. Neurobiol Dis. 2019. PMID: 31445160 Free PMC article. Review.
-
Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders.Biochem Pharmacol. 2011 Oct 15;82(8):873-82. doi: 10.1016/j.bcp.2011.06.001. Epub 2011 Jun 13. Biochem Pharmacol. 2011. PMID: 21684266 Free PMC article. Review.
References
-
- Artymyshyn R, Smith A, Wolfe BB. The use of 3H standards in 125I autoradiography. J Neurosci Methods. 1990;32:185–192. - PubMed
-
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. - PubMed
-
- Bordia T, Campos C, Huang LZ, Quik M. Continuous and intermittent nicotine treatment reduces L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327:239–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS047162-01/NS/NINDS NIH HHS/United States
- R01 NS042091-03/NS/NINDS NIH HHS/United States
- R01 NS047162-05/NS/NINDS NIH HHS/United States
- R01 NS042091-04/NS/NINDS NIH HHS/United States
- NS47162/NS/NINDS NIH HHS/United States
- R01 NS042091-02/NS/NINDS NIH HHS/United States
- R01 NS047162/NS/NINDS NIH HHS/United States
- R01 NS042091-05/NS/NINDS NIH HHS/United States
- R01 NS042091-01A2/NS/NINDS NIH HHS/United States
- R01 NS059910/NS/NINDS NIH HHS/United States
- R01 NS047162-04/NS/NINDS NIH HHS/United States
- R01 NS042091/NS/NINDS NIH HHS/United States
- R01 NS047162-02/NS/NINDS NIH HHS/United States
- R01 NS042091-06/NS/NINDS NIH HHS/United States
- NS42091/NS/NINDS NIH HHS/United States
- R01 NS047162-03/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

