Carrier testing for severe childhood recessive diseases by next-generation sequencing
- PMID: 21228398
- PMCID: PMC3740116
- DOI: 10.1126/scitranslmed.3001756
Carrier testing for severe childhood recessive diseases by next-generation sequencing
Abstract
Of 7028 disorders with suspected Mendelian inheritance, 1139 are recessive and have an established molecular basis. Although individually uncommon, Mendelian diseases collectively account for ~20% of infant mortality and ~10% of pediatric hospitalizations. Preconception screening, together with genetic counseling of carriers, has resulted in remarkable declines in the incidence of several severe recessive diseases including Tay-Sachs disease and cystic fibrosis. However, extension of preconception screening to most severe disease genes has hitherto been impractical. Here, we report a preconception carrier screen for 448 severe recessive childhood diseases. Rather than costly, complete sequencing of the human genome, 7717 regions from 437 target genes were enriched by hybrid capture or microdroplet polymerase chain reaction, sequenced by next-generation sequencing (NGS) to a depth of up to 2.7 gigabases, and assessed with stringent bioinformatic filters. At a resultant 160x average target coverage, 93% of nucleotides had at least 20x coverage, and mutation detection/genotyping had ~95% sensitivity and ~100% specificity for substitution, insertion/deletion, splicing, and gross deletion mutations and single-nucleotide polymorphisms. In 104 unrelated DNA samples, the average genomic carrier burden for severe pediatric recessive mutations was 2.8 and ranged from 0 to 7. The distribution of mutations among sequenced samples appeared random. Twenty-seven percent of mutations cited in the literature were found to be common polymorphisms or misannotated, underscoring the need for better mutation databases as part of a comprehensive carrier testing strategy. Given the magnitude of carrier burden and the lower cost of testing compared to treating these conditions, carrier screening by NGS made available to the general population may be an economical way to reduce the incidence of and ameliorate suffering associated with severe recessive childhood disorders.
Conflict of interest statement
Figures
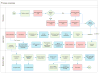
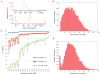
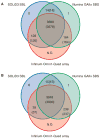
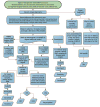



Comment in
-
Molecular technologies open new clinical genetic vistas.Sci Transl Med. 2011 Jan 12;3(65):65ps2. doi: 10.1126/scitranslmed.3002064. Sci Transl Med. 2011. PMID: 21228396
-
Sequencing: the next generation. Moving beyond population-based recessive disease carrier screening.Clin Genet. 2011 Jul;80(1):25-6. doi: 10.1111/j.1399-0004.2011.01677.x. Epub 2011 Apr 25. Clin Genet. 2011. PMID: 21476994
Similar articles
-
Comprehensive carrier screening and molecular diagnostic testing for recessive childhood diseases.PLoS Curr. 2012 May 2;4:e4f9877ab8ffa9. doi: 10.1371/4f9877ab8ffa9. PLoS Curr. 2012. PMID: 22872815 Free PMC article.
-
Screening for Tay-Sachs disease carriers by full-exon sequencing with novel variant interpretation outperforms enzyme testing in a pan-ethnic cohort.Mol Genet Genomic Med. 2019 Aug;7(8):e836. doi: 10.1002/mgg3.836. Epub 2019 Jul 10. Mol Genet Genomic Med. 2019. PMID: 31293106 Free PMC article.
-
Comprehensive carrier genetic test using next-generation deoxyribonucleic acid sequencing in infertile couples wishing to conceive through assisted reproductive technology.Fertil Steril. 2015 Nov;104(5):1286-93. doi: 10.1016/j.fertnstert.2015.07.1166. Epub 2015 Sep 3. Fertil Steril. 2015. PMID: 26354092
-
Carrier testing for autosomal-recessive disorders.Crit Rev Clin Lab Sci. 2003 Aug;40(4):473-97. doi: 10.1080/10408360390247832. Crit Rev Clin Lab Sci. 2003. PMID: 14582604 Review.
-
Variables that underlie cost efficacy of prenatal screening.Obstet Gynecol Clin North Am. 2002 Jun;29(2):277-86, v-vi. doi: 10.1016/s0889-8545(01)00007-9. Obstet Gynecol Clin North Am. 2002. PMID: 12108828 Review.
Cited by
-
Comprehensive carrier screening and molecular diagnostic testing for recessive childhood diseases.PLoS Curr. 2012 May 2;4:e4f9877ab8ffa9. doi: 10.1371/4f9877ab8ffa9. PLoS Curr. 2012. PMID: 22872815 Free PMC article.
-
Noninvasive fetal genome sequencing: a primer.Prenat Diagn. 2013 Jun;33(6):547-54. doi: 10.1002/pd.4097. Epub 2013 Apr 1. Prenat Diagn. 2013. PMID: 23553552 Free PMC article. Review.
-
EFHC1 variants in juvenile myoclonic epilepsy: reanalysis according to NHGRI and ACMG guidelines for assigning disease causality.Genet Med. 2017 Feb;19(2):144-156. doi: 10.1038/gim.2016.86. Epub 2016 Jul 28. Genet Med. 2017. PMID: 27467453
-
A genome-wide case-only test for the detection of digenic inheritance in human exomes.Proc Natl Acad Sci U S A. 2020 Aug 11;117(32):19367-19375. doi: 10.1073/pnas.1920650117. Epub 2020 Jul 27. Proc Natl Acad Sci U S A. 2020. PMID: 32719112 Free PMC article.
-
Genetic mapping and exome sequencing identify variants associated with five novel diseases.PLoS One. 2012;7(1):e28936. doi: 10.1371/journal.pone.0028936. Epub 2012 Jan 17. PLoS One. 2012. PMID: 22279524 Free PMC article.
References
-
- Kaback MM. Hexosaminidase A deficiency. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews. University of Washington; Seattle: 1993.
-
- Kronn D, Jansen V, Ostrer H. Carrier screening for cystic fibrosis, Gaucher disease, and Tay-Sachs disease in the Ashkenazi Jewish population: The first 1000 cases at New York University Medical Center, New York NY. Arch Intern Med. 1998;158:777–781. - PubMed
-
- Kaback MM. Population-based genetic screening for reproductive counseling: The Tay-Sachs disease model. Eur J Pediatr. 2000;159:S192–S195. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

