Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus
- PMID: 21228237
- PMCID: PMC3067931
- DOI: 10.1128/JVI.02065-10
Recombinant Sendai viruses expressing fusion proteins with two furin cleavage sites mimic the syncytial and receptor-independent infection properties of respiratory syncytial virus
Abstract
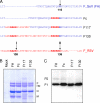
Cell entry by paramyxoviruses requires fusion between viral and cellular membranes. Paramyxovirus infection also gives rise to the formation of multinuclear, fused cells (syncytia). Both types of fusion are mediated by the viral fusion (F) protein, which requires proteolytic processing at a basic cleavage site in order to be active for fusion. In common with most paramyxoviruses, fusion mediated by Sendai virus F protein (F(SeV)) requires coexpression of the homologous attachment (hemagglutinin-neuraminidase [HN]) protein, which binds to cell surface sialic acid receptors. In contrast, respiratory syncytial virus fusion protein (F(RSV)) is capable of fusing membranes in the absence of the viral attachment (G) protein. Moreover, F(RSV) is unique among paramyxovirus fusion proteins since F(RSV) possesses two multibasic cleavage sites, which are separated by an intervening region of 27 amino acids. We have previously shown that insertion of both F(RSV) cleavage sites in F(SeV) decreases dependency on the HN attachment protein for syncytium formation in transfected cells. We now describe recombinant Sendai viruses (rSeV) that express mutant F proteins containing one or both F(RSV) cleavage sites. All cleavage-site mutant viruses displayed reduced thermostability, with double-cleavage-site mutants exhibiting a hyperfusogenic phenotype in infected cells. Furthermore, insertion of both F(RSV) cleavage sites in F(SeV) reduced dependency on the interaction of HN with sialic acid for infection, thus mimicking the unique ability of RSV to fuse and infect cells in the absence of a separate attachment protein.
Figures

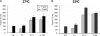
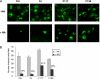
Similar articles
-
Insertion of the two cleavage sites of the respiratory syncytial virus fusion protein in Sendai virus fusion protein leads to enhanced cell-cell fusion and a decreased dependency on the HN attachment protein for activity.J Virol. 2008 Jun;82(12):5986-98. doi: 10.1128/JVI.00078-08. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385247 Free PMC article.
-
A chimeric respiratory syncytial virus fusion protein functionally replaces the F and HN glycoproteins in recombinant Sendai virus.J Virol. 2005 Aug;79(16):10467-77. doi: 10.1128/JVI.79.16.10467-10477.2005. J Virol. 2005. PMID: 16051839 Free PMC article.
-
Sendai virus recombinant vaccine expressing hPIV-3 HN or F elicits protective immunity and combines with a second recombinant to prevent hPIV-1, hPIV-3 and RSV infections.Vaccine. 2008 Jun 25;26(27-28):3480-8. doi: 10.1016/j.vaccine.2008.04.022. Epub 2008 May 1. Vaccine. 2008. PMID: 18499307 Free PMC article.
-
[Research Progress in Membrane Fusion of the Respiratory Syncytial Virus].Bing Du Xue Bao. 2015 Sep;31(5):565-72. Bing Du Xue Bao. 2015. PMID: 26738297 Review. Chinese.
-
Structure and function of a paramyxovirus fusion protein.Biochim Biophys Acta. 2003 Jul 11;1614(1):73-84. doi: 10.1016/s0005-2736(03)00164-0. Biochim Biophys Acta. 2003. PMID: 12873767 Review.
Cited by
-
The RSV F p27 peptide: current knowledge, important questions.Front Microbiol. 2023 Jun 21;14:1219846. doi: 10.3389/fmicb.2023.1219846. eCollection 2023. Front Microbiol. 2023. PMID: 37415824 Free PMC article. Review.
-
Residues of the human metapneumovirus fusion (F) protein critical for its strain-related fusion phenotype: implications for the virus replication cycle.J Virol. 2011 Dec;85(23):12650-61. doi: 10.1128/JVI.05485-11. Epub 2011 Sep 21. J Virol. 2011. PMID: 21937649 Free PMC article.
-
Use of single chain antibody derivatives for targeted drug delivery.Mol Med. 2016 Sep;22:258-270. doi: 10.2119/molmed.2016.00043. Epub 2016 Apr 22. Mol Med. 2016. PMID: 27249008 Free PMC article.
-
Entry of enveloped viruses into host cells: membrane fusion.Subcell Biochem. 2013;68:467-87. doi: 10.1007/978-94-007-6552-8_16. Subcell Biochem. 2013. PMID: 23737062 Free PMC article. Review.
-
Novel requirements for HAP2/GCS1-mediated gamete fusion in Tetrahymena.iScience. 2024 May 28;27(6):110146. doi: 10.1016/j.isci.2024.110146. eCollection 2024 Jun 21. iScience. 2024. PMID: 38904066 Free PMC article.
References
-
- Borisevich, V., et al. 2008. A highly sensitive and versatile virus titration assay in the 96-well microplate format. J. Virol. Methods 147:197-205. - PubMed
-
- Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

