Amantadine for dyskinesias in Parkinson's disease: a randomized controlled trial
- PMID: 21217832
- PMCID: PMC3013111
- DOI: 10.1371/journal.pone.0015298
Amantadine for dyskinesias in Parkinson's disease: a randomized controlled trial
Abstract
Background: Dyskinesias are some of the major motor complications that impair quality of life for patients with Parkinson's disease. The purpose of the present study was to investigate the efficacy of amantadine in Parkinson's disease patients suffering from dyskinesias.
Methods: In this multi-center, double-blind, randomized, placebo-controlled, cross-over trial, 36 patients with Parkinson's disease and dyskinesias were randomized, and 62 interventions, which included amantadine (300 mg/day) or placebo treatment for 27 days, were analyzed. At 15 days after washout, the treatments were crossed over. The primary outcome measure was the changes in the Rush Dyskinesia Rating Scale (RDRS) during each treatment period. The secondary outcome measures were changes in the Unified Parkinson's Disease Rating Scale part IVa (UPDRS-IVa, dyskinesias), part IVb (motor fluctuations), and part III (motor function).
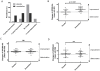
Results: RDRS improved in 64% and 16% of patients treated with amantadine or placebo, respectively, with significant differences between treatments. The adjusted odds-ratio for improvement by amantadine was 6.7 (95% confidence interval, 1.4 to 31.5). UPDRS-IVa was improved to a significantly greater degree in amantadine-treated patients [mean (SD) of 1.83 (1.56)] compared with placebo-treated patients [0.03 (1.51)]. However, there were no significant effects on UPDRS-IVb or III scores.
Conclusions: Results from the present study demonstrated that amantadine exhibited efficacious effects against dyskinesias in 60-70% of patients.
Trial registration: UMIN Clinical Trial Registry UMIN000000780.
Conflict of interest statement
Figures
Similar articles
-
Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson's disease.Neurology. 1998 May;50(5):1323-6. doi: 10.1212/wnl.50.5.1323. Neurology. 1998. PMID: 9595981 Clinical Trial.
-
Amantadine for dyskinesia in Parkinson's disease.Cochrane Database Syst Rev. 2003;2003(2):CD003467. doi: 10.1002/14651858.CD003467. Cochrane Database Syst Rev. 2003. PMID: 12804468 Free PMC article. Review.
-
Impact of dyskinesia on activities of daily living in Parkinson's disease: Results from pooled phase 3 ADS-5102 clinical trials.Parkinsonism Relat Disord. 2019 Mar;60:118-125. doi: 10.1016/j.parkreldis.2018.09.005. Epub 2018 Sep 5. Parkinsonism Relat Disord. 2019. PMID: 30292734 Clinical Trial.
-
Effect of Repeated Intravenous Amantadine Infusions in Patients with Parkinson's Disease: An Open-Label Pilot Study.Clin Transl Sci. 2019 Nov;12(6):586-590. doi: 10.1111/cts.12684. Epub 2019 Sep 24. Clin Transl Sci. 2019. PMID: 31436382 Free PMC article. Clinical Trial.
-
Ropinirole for levodopa-induced complications in Parkinson's disease.Cochrane Database Syst Rev. 2001;(1):CD001516. doi: 10.1002/14651858.CD001516. Cochrane Database Syst Rev. 2001. PMID: 11279718 Review.
Cited by
-
Non-dopamine receptor ligands for the treatment of Parkinson's disease. Insight into the related chemical/property space.Mol Divers. 2016 Feb;20(1):345-65. doi: 10.1007/s11030-015-9598-y. Epub 2015 May 9. Mol Divers. 2016. PMID: 25956815 Review.
-
Contemporary Options for the Management of Motor Complications in Parkinson's Disease: Updated Clinical Review.Drugs. 2019 Apr;79(6):593-608. doi: 10.1007/s40265-019-01098-w. Drugs. 2019. PMID: 30905034 Review.
-
Metabotropic Glutamate Receptor 4 (mGlu4) Positive Allosteric Modulators Lack Efficacy in Rat and Marmoset Models of L-DOPA-Induced Dyskinesia.J Parkinsons Dis. 2024;14(2):245-259. doi: 10.3233/JPD-230296. J Parkinsons Dis. 2024. PMID: 38427500 Free PMC article.
-
Drug safety profiles in geriatric patients with Parkinson's disease using the FORTA (Fit fOR The Aged) classification: results from a mono-centric retrospective analysis.J Neural Transm (Vienna). 2021 Jan;128(1):49-60. doi: 10.1007/s00702-020-02276-x. Epub 2020 Dec 1. J Neural Transm (Vienna). 2021. PMID: 33263172 Free PMC article.
-
Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson's disease: a dose-finding study.Brain. 2015 Apr;138(Pt 4):963-73. doi: 10.1093/brain/awu409. Epub 2015 Feb 10. Brain. 2015. PMID: 25669730 Free PMC article. Clinical Trial.
References
-
- de Rijk MC, Tzourio C, Breteler MM, Dartigues JF, Amaducci L, et al. Prevalence of parkinsonism and Parkinson's disease in Europe: the EUROPARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;62:10–15. - PMC - PubMed
-
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol. 2009;8:67–81. - PubMed
-
- Chapuis S, Ouchchane L, Metz O, Gerbaud L, Durif F. Impact of the motor complications of Parkinson's disease on the quality of life. Mov Disord. 2005;20:224–230. - PubMed
-
- Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

