The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance
- PMID: 21217695
- PMCID: PMC3076025
- DOI: 10.1038/nm.2279
The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance
Abstract
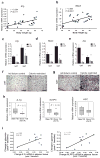
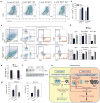
The emergence of chronic inflammation during obesity in the absence of overt infection or well-defined autoimmune processes is a puzzling phenomenon. The Nod-like receptor (NLR) family of innate immune cell sensors, such as the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (Nlrp3, but also known as Nalp3 or cryopyrin) inflammasome are implicated in recognizing certain nonmicrobial originated 'danger signals' leading to caspase-1 activation and subsequent interleukin-1β (IL-1β) and IL-18 secretion. We show that calorie restriction and exercise-mediated weight loss in obese individuals with type 2 diabetes is associated with a reduction in adipose tissue expression of Nlrp3 as well as with decreased inflammation and improved insulin sensitivity. We further found that the Nlrp3 inflammasome senses lipotoxicity-associated increases in intracellular ceramide to induce caspase-1 cleavage in macrophages and adipose tissue. Ablation of Nlrp3 in mice prevents obesity-induced inflammasome activation in fat depots and liver as well as enhances insulin signaling. Furthermore, elimination of Nlrp3 in obese mice reduces IL-18 and adipose tissue interferon-γ (IFN-γ) expression, increases naive T cell numbers and reduces effector T cell numbers in adipose tissue. Collectively, these data establish that the Nlrp3 inflammasome senses obesity-associated danger signals and contributes to obesity-induced inflammation and insulin resistance.
Figures




Comment in
-
Linking the inflammasome to obesity-related disease.Nat Med. 2011 Feb;17(2):164-5. doi: 10.1038/nm0211-164. Nat Med. 2011. PMID: 21297609 No abstract available.
Similar articles
-
A casein hydrolysate protects mice against high fat diet induced hyperglycemia by attenuating NLRP3 inflammasome-mediated inflammation and improving insulin signaling.Mol Nutr Food Res. 2016 Nov;60(11):2421-2432. doi: 10.1002/mnfr.201501054. Epub 2016 Sep 8. Mol Nutr Food Res. 2016. PMID: 27390025
-
Suppression of NLRP3 inflammasome by γ-tocotrienol ameliorates type 2 diabetes.J Lipid Res. 2016 Jan;57(1):66-76. doi: 10.1194/jlr.M062828. Epub 2015 Dec 1. J Lipid Res. 2016. PMID: 26628639 Free PMC article.
-
Macrophage-specific de Novo Synthesis of Ceramide Is Dispensable for Inflammasome-driven Inflammation and Insulin Resistance in Obesity.J Biol Chem. 2015 Dec 4;290(49):29402-13. doi: 10.1074/jbc.M115.680199. Epub 2015 Oct 5. J Biol Chem. 2015. PMID: 26438821 Free PMC article.
-
The NLRP3 inflammasome regulates adipose tissue metabolism.Biochem J. 2020 Mar 27;477(6):1089-1107. doi: 10.1042/BCJ20190472. Biochem J. 2020. PMID: 32202638 Review.
-
Inflammation links excess fat to insulin resistance: the role of the interleukin-1 family.Immunol Rev. 2012 Sep;249(1):239-52. doi: 10.1111/j.1600-065X.2012.01145.x. Immunol Rev. 2012. PMID: 22889226 Review.
Cited by
-
Blockade of the Platelet-Driven CXCL7-CXCR1/2 Inflammatory Axis Prevents Murine Cerebral Aneurysm Formation and Rupture.Transl Stroke Res. 2024 Nov 5. doi: 10.1007/s12975-024-01304-2. Online ahead of print. Transl Stroke Res. 2024. PMID: 39499487
-
Association between changes in body composition and progression of liver fibrosis in patients with type 2 diabetes mellitus.Front Nutr. 2024 Oct 21;11:1476467. doi: 10.3389/fnut.2024.1476467. eCollection 2024. Front Nutr. 2024. PMID: 39498408 Free PMC article.
-
Unveiling the nexus: pyroptosis and its crucial implications in liver diseases.Mol Cell Biochem. 2024 Oct 31. doi: 10.1007/s11010-024-05147-1. Online ahead of print. Mol Cell Biochem. 2024. PMID: 39477911 Review.
-
The role of moderate to vigorous physical activity level and number of treatments/medications in mediating the effect of body mass index on diabetic nephropathy: a Mendelian randomization study.Ren Fail. 2024 Dec;46(2):2417738. doi: 10.1080/0886022X.2024.2417738. Epub 2024 Oct 28. Ren Fail. 2024. PMID: 39466707 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

