Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease
- PMID: 21209324
- PMCID: PMC3046053
- DOI: 10.1091/mbc.E10-08-0718
Mitochondrial enzymes are protected from stress-induced aggregation by mitochondrial chaperones and the Pim1/LON protease
Abstract
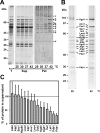
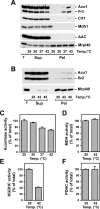
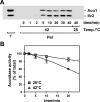
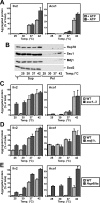
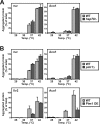
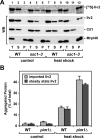
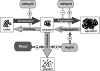
Proteins in a natural environment are constantly challenged by stress conditions, causing their destabilization, unfolding, and, ultimately, aggregation. Protein aggregation has been associated with a wide variety of pathological conditions, especially neurodegenerative disorders, stressing the importance of adequate cellular protein quality control measures to counteract aggregate formation. To secure protein homeostasis, mitochondria contain an elaborate protein quality control system, consisting of chaperones and ATP-dependent proteases. To determine the effects of protein aggregation on the functional integrity of mitochondria, we set out to identify aggregation-prone endogenous mitochondrial proteins. We could show that major metabolic pathways in mitochondria were affected by the aggregation of key enzyme components, which were largely inactivated after heat stress. Furthermore, treatment with elevated levels of reactive oxygen species strongly influenced the aggregation behavior, in particular in combination with elevated temperatures. Using specific chaperone mutant strains, we showed a protective effect of the mitochondrial Hsp70 and Hsp60 chaperone systems. Moreover, accumulation of aggregated polypeptides was strongly decreased by the AAA-protease Pim1/LON. We therefore propose that the proteolytic breakdown of aggregation-prone polypeptides represents a major protective strategy to prevent the in vivo formation of aggregates in mitochondria.
Figures

Similar articles
-
Mitochondrial protein homeostasis: the cooperative roles of chaperones and proteases.Res Microbiol. 2009 Nov;160(9):718-25. doi: 10.1016/j.resmic.2009.08.003. Epub 2009 Aug 31. Res Microbiol. 2009. PMID: 19723579 Review.
-
Molecular chaperones cooperate with PIM1 protease in the degradation of misfolded proteins in mitochondria.EMBO J. 1994 Nov 1;13(21):5135-45. doi: 10.1002/j.1460-2075.1994.tb06843.x. EMBO J. 1994. PMID: 7957078 Free PMC article.
-
Substitution of PIM1 protease in mitochondria by Escherichia coli Lon protease.J Biol Chem. 1996 Apr 26;271(17):10137-42. doi: 10.1074/jbc.271.17.10137. J Biol Chem. 1996. PMID: 8626573
-
Identification of novel oxidized protein substrates and physiological partners of the mitochondrial ATP-dependent Lon-like protease Pim1.J Biol Chem. 2010 Apr 9;285(15):11445-57. doi: 10.1074/jbc.M109.065425. Epub 2010 Feb 11. J Biol Chem. 2010. PMID: 20150421 Free PMC article.
-
ATP-dependent proteases controlling mitochondrial function in the yeast Saccharomyces cerevisiae.Cell Mol Life Sci. 1999 Nov 30;56(9-10):825-42. doi: 10.1007/s000180050029. Cell Mol Life Sci. 1999. PMID: 11212342 Free PMC article. Review.
Cited by
-
Mitochondrial Quality Control: Its Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).J Obes Metab Syndr. 2023 Dec 30;32(4):289-302. doi: 10.7570/jomes23054. Epub 2023 Dec 5. J Obes Metab Syndr. 2023. PMID: 38049180 Free PMC article. Review.
-
Mild photothermal therapy assist in promoting bone repair: Related mechanism and materials.Mater Today Bio. 2023 Oct 20;23:100834. doi: 10.1016/j.mtbio.2023.100834. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38024841 Free PMC article. Review.
-
Immunoproteasome-specific subunit PSMB9 induction is required to regulate cellular proteostasis upon mitochondrial dysfunction.Nat Commun. 2023 Jul 11;14(1):4092. doi: 10.1038/s41467-023-39642-8. Nat Commun. 2023. PMID: 37433777 Free PMC article.
-
Mitochondrial complexome reveals quality-control pathways of protein import.Nature. 2023 Feb;614(7946):153-159. doi: 10.1038/s41586-022-05641-w. Epub 2023 Jan 25. Nature. 2023. PMID: 36697829 Free PMC article.
-
The mitochondrial Hsp70 controls the assembly of the F1FO-ATP synthase.Nat Commun. 2023 Jan 3;14(1):39. doi: 10.1038/s41467-022-35720-5. Nat Commun. 2023. PMID: 36596815 Free PMC article.
References
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Bateman JM, Iacovino M, Perlman PS, Butow RA. Mitochondrial DNA instability mutants of the bifunctional protein Ilv5p have altered organization in mitochondria and are targeted for degradation by Hsp78 and the Pim1p protease. J Biol Chem. 2002;277:47946–47953. - PubMed
-
- Bender T, Leidhold C, Ruppert T, Franken S, Voos W. The role of protein quality control in mitochondrial protein homeostasis under oxidative stress. Proteomics. 2010;10:1426–1443. - PubMed
-
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

