Copper resistance is essential for virulence of Mycobacterium tuberculosis
- PMID: 21205886
- PMCID: PMC3029754
- DOI: 10.1073/pnas.1009261108
Copper resistance is essential for virulence of Mycobacterium tuberculosis
Abstract
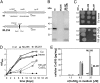
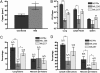
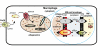
Copper (Cu) is essential for many biological processes, but is toxic when present in excessive amounts. In this study, we provide evidence that Cu plays a crucial role in controlling tuberculosis. A Mycobacterium tuberculosis (Mtb) mutant lacking the outer membrane channel protein Rv1698 accumulated 100-fold more Cu and was more susceptible to Cu toxicity than WT Mtb. Similar phenotypes were observed for a M. smegmatis mutant lacking the homolog Ms3747, demonstrating that these mycobacterial copper transport proteins B (MctB) are essential for Cu resistance and maintenance of low intracellular Cu levels. Guinea pigs responded to infection with Mtb by increasing the Cu concentration in lung lesions. Loss of MctB resulted in a 1,000- and 100-fold reduced bacterial burden in lungs and lymph nodes, respectively, in guinea pigs infected with Mtb. In mice, the persistence defect of the Mtb mctB mutant was exacerbated by the addition of Cu to the diet. These experiments provide evidence that Cu is used by the mammalian host to control Mtb infection and that Cu resistance mechanisms are crucial for Mtb virulence. Importantly, Mtb is much more susceptible to Cu than other bacteria and is killed in vitro by Cu concentrations lower than those found in phagosomes of macrophages. Hence, this study reveals an Achilles heel of Mtb that might be a promising target for tuberculosis chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
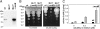
Similar articles
-
Discovery of a siderophore export system essential for virulence of Mycobacterium tuberculosis.PLoS Pathog. 2013 Jan;9(1):e1003120. doi: 10.1371/journal.ppat.1003120. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23431276 Free PMC article.
-
PPE11 of Mycobacterium tuberculosis can alter host inflammatory response and trigger cell death.Microb Pathog. 2019 Jan;126:45-55. doi: 10.1016/j.micpath.2018.10.031. Epub 2018 Oct 23. Microb Pathog. 2019. PMID: 30366125
-
Mycobacterium tuberculosis Rv1324 Protein Contributes to Mycobacterial Persistence and Causes Pathological Lung Injury in Mice by Inducing Ferroptosis.Microbiol Spectr. 2023 Feb 14;11(1):e0252622. doi: 10.1128/spectrum.02526-22. Epub 2023 Jan 10. Microbiol Spectr. 2023. PMID: 36625672 Free PMC article.
-
Resistance mechanisms of Mycobacterium tuberculosis against phagosomal copper overload.Tuberculosis (Edinb). 2012 May;92(3):202-10. doi: 10.1016/j.tube.2011.12.006. Epub 2012 Feb 22. Tuberculosis (Edinb). 2012. PMID: 22361385 Free PMC article. Review.
-
Copper homeostasis in Mycobacterium tuberculosis.Metallomics. 2015 Jun;7(6):929-34. doi: 10.1039/c4mt00305e. Metallomics. 2015. PMID: 25614981 Free PMC article. Review.
Cited by
-
Copper at the Fungal Pathogen-Host Axis.J Biol Chem. 2015 Jul 31;290(31):18945-53. doi: 10.1074/jbc.R115.649129. Epub 2015 Jun 8. J Biol Chem. 2015. PMID: 26055724 Free PMC article. Review.
-
Copper Resistance of the Emerging Pathogen Acinetobacter baumannii.Appl Environ Microbiol. 2016 Sep 30;82(20):6174-6188. doi: 10.1128/AEM.01813-16. Print 2016 Oct 15. Appl Environ Microbiol. 2016. PMID: 27520808 Free PMC article.
-
FKS1 Is Required for Cryptococcus neoformans Fitness In Vivo: Application of Copper-Regulated Gene Expression to Mouse Models of Cryptococcosis.mSphere. 2022 Jun 29;7(3):e0016322. doi: 10.1128/msphere.00163-22. Epub 2022 May 4. mSphere. 2022. PMID: 35506343 Free PMC article.
-
Essential trace elemental levels (zinc, iron and copper) in the biological samples of smoker referent and pulmonary tuberculosis patients.Toxicol Rep. 2019 Nov 15;6:1230-1239. doi: 10.1016/j.toxrep.2019.11.011. eCollection 2019. Toxicol Rep. 2019. PMID: 31799123 Free PMC article.
-
Assessment of Haloferax mediterranei Genome in Search of Copper-Molecular Machinery With Potential Applications for Bioremediation.Front Microbiol. 2022 Jun 15;13:895296. doi: 10.3389/fmicb.2022.895296. eCollection 2022. Front Microbiol. 2022. PMID: 35783429 Free PMC article.
References
-
- Crichton RR, Pierre JL. Old iron, young copper: From Mars to Venus. Biometals. 2001;14:99–112. - PubMed
-
- Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: The requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. - PubMed
-
- Prohaska JR, Lukasewycz OA. Copper deficiency suppresses the immune response of mice. Science. 1981;213:559–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

