Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii
- PMID: 21203588
- PMCID: PMC3006384
- DOI: 10.1371/journal.pbio.1000576
Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii
Erratum in
-
Correction: Phosphorylation of Mouse Immunity-Related GTPase (IRG) Resistance Proteins Is an Evasion Strategy for Virulent Toxoplasma gondii.PLoS Biol. 2015 Jul 9;13(7):e1002199. doi: 10.1371/journal.pbio.1002199. eCollection 2015 Jul. PLoS Biol. 2015. PMID: 26158675 Free PMC article. No abstract available.
Abstract
Virulence of complex pathogens in mammals is generally determined by multiple components of the pathogen interacting with the functional complexity and multiple layering of the mammalian immune system. It is most unusual for the resistance of a mammalian host to be overcome by the defeat of a single defence mechanism. In this study we uncover and analyse just such a case at the molecular level, involving the widespread intracellular protozoan pathogen Toxoplasma gondii and one of its most important natural hosts, the house mouse (Mus musculus). Natural polymorphism in virulence of Eurasian T. gondii strains for mice has been correlated in genetic screens with the expression of polymorphic rhoptry kinases (ROP kinases) secreted into the host cell during infection. We show that the molecular targets of the virulent allelic form of ROP18 kinase are members of a family of cellular GTPases, the interferon-inducible IRG (immunity-related GTPase) proteins, known from earlier work to be essential resistance factors in mice against avirulent strains of T. gondii. Virulent T. gondii strain ROP18 kinase phosphorylates several mouse IRG proteins. We show that the parasite kinase phosphorylates host Irga6 at two threonines in the nucleotide-binding domain, biochemically inactivating the GTPase and inhibiting its accumulation and action at the T. gondii parasitophorous vacuole membrane. Our analysis identifies the conformationally active switch I region of the GTP-binding site as an Achilles' heel of the IRG protein pathogen-resistance mechanism. The polymorphism of ROP18 in natural T. gondii populations indicates the existence of a dynamic, rapidly evolving ecological relationship between parasite virulence factors and host resistance factors. This system should be unusually fruitful for analysis at both ecological and molecular levels since both T. gondii and the mouse are widespread and abundant in the wild and are well-established model species with excellent analytical tools available.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



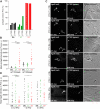
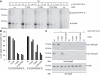
Similar articles
-
The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7.Cell Microbiol. 2016 Feb;18(2):244-59. doi: 10.1111/cmi.12499. Epub 2015 Oct 30. Cell Microbiol. 2016. PMID: 26247512 Free PMC article.
-
A Toxoplasma gondii pseudokinase inhibits host IRG resistance proteins.PLoS Biol. 2012;10(7):e1001358. doi: 10.1371/journal.pbio.1001358. Epub 2012 Jul 10. PLoS Biol. 2012. PMID: 22802726 Free PMC article.
-
Toxoplasma gondii GRA60 is an effector protein that modulates host cell autonomous immunity and contributes to virulence.Cell Microbiol. 2021 Feb;23(2):e13278. doi: 10.1111/cmi.13278. Epub 2020 Oct 23. Cell Microbiol. 2021. PMID: 33040458
-
Toxoplasma gondii and the Immunity-Related GTPase (IRG) resistance system in mice: a review.Mem Inst Oswaldo Cruz. 2009 Mar;104(2):234-40. doi: 10.1590/s0074-02762009000200016. Mem Inst Oswaldo Cruz. 2009. PMID: 19430648 Review.
-
Decoding Toxoplasma gondii virulence: the mechanisms of IRG protein inactivation.Trends Parasitol. 2024 Sep;40(9):805-819. doi: 10.1016/j.pt.2024.07.009. Epub 2024 Aug 20. Trends Parasitol. 2024. PMID: 39168720 Review.
Cited by
-
Secretion of Rhoptry and Dense Granule Effector Proteins by Nonreplicating Toxoplasma gondii Uracil Auxotrophs Controls the Development of Antitumor Immunity.PLoS Genet. 2016 Jul 22;12(7):e1006189. doi: 10.1371/journal.pgen.1006189. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27447180 Free PMC article.
-
The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response.PLoS Pathog. 2012;8(6):e1002784. doi: 10.1371/journal.ppat.1002784. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761577 Free PMC article.
-
Rhoptry and Dense Granule Secreted Effectors Regulate CD8+ T Cell Recognition of Toxoplasma gondii Infected Host Cells.Front Immunol. 2019 Sep 6;10:2104. doi: 10.3389/fimmu.2019.02104. eCollection 2019. Front Immunol. 2019. PMID: 31555296 Free PMC article.
-
Interplay Between Toxoplasma gondii, Autophagy, and Autophagy Proteins.Front Cell Infect Microbiol. 2019 May 1;9:139. doi: 10.3389/fcimb.2019.00139. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31119109 Free PMC article. Review.
-
Identification of three novel Toxoplasma gondii rhoptry proteins.Int J Parasitol. 2014 Feb;44(2):147-60. doi: 10.1016/j.ijpara.2013.08.002. Epub 2013 Sep 24. Int J Parasitol. 2014. PMID: 24070999 Free PMC article.
References
-
- Dubey J. The life cycle of Toxoplasma gondii. In: Ajioka J. W, Soldati D, editors. Toxoplasma Molecular and Cellular Biology. Norfolk, UK: Horizon Bioscience; 2007. pp. 3–16.
-
- Howe D. K, Sibley L. D. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. - PubMed
-
- Sibley L. D, Ajioka J. W. Population structure of Toxoplasma gondii: Clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol. 2008;62:329–351. - PubMed
-
- Sibley L. D, Boothroyd J. C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. - PubMed
-
- El Hajj H, Lebrun M, Arold S. T, Vial H, Labesse G, et al. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

