Therapeutic cancer vaccines: are we there yet?
- PMID: 21198663
- PMCID: PMC3075547
- DOI: 10.1111/j.1600-065X.2010.00979.x
Therapeutic cancer vaccines: are we there yet?
Abstract
Enthusiasm for therapeutic cancer vaccines has been rejuvenated with the recent completion of several large, randomized phase III clinical trials that in some cases have reported an improvement in progression free or overall survival. However, an honest appraisal of their efficacy reveals modest clinical benefit and a frequent requirement for patients with relatively indolent cancers and minimal or no measurable disease. Experience with adoptive cell transfer-based immunotherapies unequivocally establishes that T cells can mediate durable complete responses, even in the setting of advanced metastatic disease. Further, these findings reveal that the successful vaccines of the future must confront: (i) a corrupted tumor microenvironment containing regulatory T cells and aberrantly matured myeloid cells, (ii) a tumor-specific T-cell repertoire that is prone to immunologic exhaustion and senescence, and (iii) highly mutable tumor targets capable of antigen loss and immune evasion. Future progress may come from innovations in the development of selective preparative regimens that eliminate or neutralize suppressive cellular populations, more effective immunologic adjuvants, and further refinement of agents capable of antagonizing immune check-point blockade pathways.
© 2010 John Wiley & Sons A/S.
Figures
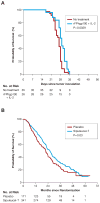
Similar articles
-
Recent developments in cancer vaccines.J Immunol. 2011 Feb 1;186(3):1325-31. doi: 10.4049/jimmunol.0902539. J Immunol. 2011. PMID: 21248270 Free PMC article. Review.
-
Tumor-specific antigens and immunologic adjuvants in cancer immunotherapy.Cancer J. 2011 Sep-Oct;17(5):325-30. doi: 10.1097/PPO.0b013e3182326004. Cancer J. 2011. PMID: 21952282 Review.
-
Enhancing immune responses to tumor-associated antigens.Cancer Biol Ther. 2009 Aug;8(15):1440-9. doi: 10.4161/cbt.8.15.9133. Epub 2009 Aug 1. Cancer Biol Ther. 2009. PMID: 19556848 Free PMC article. Review.
-
Optimization of Peptide Vaccines to Induce Robust Antitumor CD4 T-cell Responses.Cancer Immunol Res. 2017 Jan;5(1):72-83. doi: 10.1158/2326-6066.CIR-16-0194. Epub 2016 Dec 9. Cancer Immunol Res. 2017. PMID: 27941004 Free PMC article.
-
Strategies for immunotherapy of cancer.Adv Immunol. 2000;75:235-82. doi: 10.1016/s0065-2776(00)75006-1. Adv Immunol. 2000. PMID: 10879286 Review. No abstract available.
Cited by
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
-
Immunotherapy in colorectal cancer: for the select few or all?J Gastrointest Oncol. 2018 Feb;9(1):170-179. doi: 10.21037/jgo.2017.06.10. J Gastrointest Oncol. 2018. PMID: 29564183 Free PMC article. Review.
-
A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients.J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. Epub 2014 Nov 11. J Immunol Res. 2014. PMID: 25436215 Free PMC article.
-
Informatics for cancer immunotherapy.Ann Oncol. 2017 Dec 1;28(suppl_12):xii56-xii73. doi: 10.1093/annonc/mdx682. Ann Oncol. 2017. PMID: 29253114 Free PMC article. Review.
-
Telomerase and CD4 T Cell Immunity in Cancer.Cancers (Basel). 2020 Jun 25;12(6):1687. doi: 10.3390/cancers12061687. Cancers (Basel). 2020. PMID: 32630460 Free PMC article. Review.
References
-
- Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. - PubMed
-
- Paavonen J, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. - PubMed
-
- Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. - PubMed
-
- Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

