Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation
- PMID: 21194227
- PMCID: PMC3024462
- DOI: 10.1021/jm1012787
Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation
Abstract
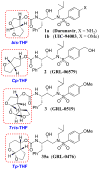
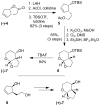
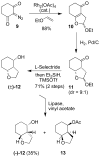
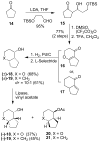
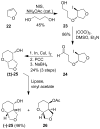
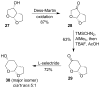
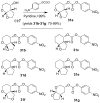
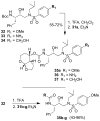
The design, synthesis, and evaluation of a new series of hexahydrofuropyranol-derived HIV-1 protease inhibitors are described. We have designed a stereochemically defined hexahydrofuropyranol-derived urethane as the P2-ligand. The current ligand is designed based upon the X-ray structure of 1a-bound HIV-1 protease. The synthesis of (3aS,4S,7aR)-hexahydro-2H-furo[2,3-b]pyran-4-ol, (-)-7, was carried out in optically active form. Incorporation of this ligand provided inhibitor 35a, which has shown excellent enzyme inhibitory activity and antiviral potency. Our structure-activity studies have indicated that the stereochemistry and the position of oxygens in the ligand are important to the observed potency of the inhibitor. Inhibitor 35a has maintained excellent potency against multidrug-resistant HIV-1 variants. An active site model of 35a was created based upon the X-ray structure of 1b-bound HIV-1 protease. The model offers molecular insights regarding ligand-binding site interactions of the hexahydrofuropyranol-derived novel P2-ligand.
Figures

Similar articles
-
Structure-based design of potent HIV-1 protease inhibitors with modified P1-biphenyl ligands: synthesis, biological evaluation, and enzyme-inhibitor X-ray structural studies.J Med Chem. 2015 Jul 9;58(13):5334-43. doi: 10.1021/acs.jmedchem.5b00676. Epub 2015 Jun 24. J Med Chem. 2015. PMID: 26107245 Free PMC article.
-
Potent HIV-1 protease inhibitors incorporating meso-bicyclic urethanes as P2-ligands: structure-based design, synthesis, biological evaluation and protein-ligand X-ray studies.Org Biomol Chem. 2008 Oct 21;6(20):3703-13. doi: 10.1039/b809178a. Epub 2008 Aug 11. Org Biomol Chem. 2008. PMID: 18843400 Free PMC article.
-
Design and Synthesis of Highly Potent HIV-1 Protease Inhibitors Containing Tricyclic Fused Ring Systems as Novel P2 Ligands: Structure-Activity Studies, Biological and X-ray Structural Analysis.J Med Chem. 2018 May 24;61(10):4561-4577. doi: 10.1021/acs.jmedchem.8b00298. Epub 2018 May 15. J Med Chem. 2018. PMID: 29763303 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Resilience to resistance of HIV-1 protease inhibitors: profile of darunavir.AIDS Rev. 2008 Jul-Sep;10(3):131-42. AIDS Rev. 2008. PMID: 18820715 Free PMC article. Review.
Cited by
-
Structural Analysis of Potent Hybrid HIV-1 Protease Inhibitors Containing Bis-tetrahydrofuran in a Pseudosymmetric Dipeptide Isostere.J Med Chem. 2020 Aug 13;63(15):8296-8313. doi: 10.1021/acs.jmedchem.0c00529. Epub 2020 Aug 3. J Med Chem. 2020. PMID: 32672965 Free PMC article.
-
Substrate envelope-designed potent HIV-1 protease inhibitors to avoid drug resistance.Chem Biol. 2013 Sep 19;20(9):1116-24. doi: 10.1016/j.chembiol.2013.07.014. Epub 2013 Sep 5. Chem Biol. 2013. PMID: 24012370 Free PMC article.
-
Structure-based design, synthesis, X-ray studies, and biological evaluation of novel HIV-1 protease inhibitors containing isophthalamide-derived P2-ligands.Bioorg Med Chem Lett. 2015 Nov 1;25(21):4903-4909. doi: 10.1016/j.bmcl.2015.05.052. Epub 2015 May 30. Bioorg Med Chem Lett. 2015. PMID: 26096678 Free PMC article.
-
Investigational protease inhibitors as antiretroviral therapies.Expert Opin Investig Drugs. 2016 Oct;25(10):1189-200. doi: 10.1080/13543784.2016.1212837. Epub 2016 Aug 2. Expert Opin Investig Drugs. 2016. PMID: 27415449 Free PMC article. Review.
-
Lewis Acid Mediated Cyclizations: Diastereoselective Synthesis of Six- to Eight-Membered Substituted Cyclic Ethers.Synthesis (Stuttg). 2017 Sep;49(18):4229-4246. doi: 10.1055/s-0036-1589054. Epub 2017 Jul 25. Synthesis (Stuttg). 2017. PMID: 29983455 Free PMC article.
References
-
- Conway B. HAART in Treatment-experienced Patients in the 21st Century: the Audacity of Hope. Future Virol. 2009;4:39–41.
-
- Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview on the Effectiveness of Triple Combination Therapy in Antiretroviral-naive HIV-1 Infected Adults. AIDS. 2001;15:1369–1377. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, Weinstein MC, Freedberg KA. The Survival Benefits of AIDS Treatment in the United States. J Infec Dis. 2006;194:11–19. - PubMed
-
- Sepkowitz KA. AIDS — The First 20 Years. N Engl J Med. 2001;344:1764–1772. - PubMed
-
- Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining Morbidity and Mortality among Patients with Advanced Human Immunodeficiency Virus Infection. N Engl J Med. 1998;338:853–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

