EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells
- PMID: 21193573
- PMCID: PMC3027174
- DOI: 10.1105/tpc.110.080697
EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells
Abstract
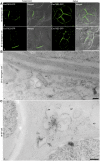
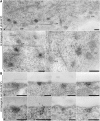
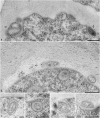
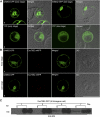
The exocyst protein complex mediates vesicle fusion with the plasma membrane. By expressing an (X)FP-tagged Arabidopsis thaliana homolog of the exocyst protein Exo70 in suspension-cultured Arabidopsis and tobacco (Nicotiana tabacum) BY-2 cells, and using antibodies specific for Exo70, we detected a compartment, which we term EXPO (for exocyst positive organelles). Standard markers for the Golgi apparatus, the trans-Golgi network/early endosome, and the multivesicular body/late endosome in plants do not colocalize with EXPO. Inhibitors of the secretory and endocytic pathways also do not affect EXPO. Exo70E2-(X)FP also locates to the plasma membrane (PM) as discrete punctae and is secreted outside of the cells. Immunogold labeling of sections cut from high-pressure frozen samples reveal EXPO to be spherical double membrane structures resembling autophagosomes. However, unlike autophagosomes, EXPOs are not induced by starvation and do not fuse with the lytic compartment or with endosomes. Instead, they fuse with the PM, releasing a single membrane vesicle into the cell wall. EXPOs are also found in other cell types, including root tips, root hair cells, and pollen grains. EXPOs therefore represent a form of unconventional secretion unique to plants.
Figures


Similar articles
-
Exocyst-Positive Organelles and Autophagosomes Are Distinct Organelles in Plants.Plant Physiol. 2015 Nov;169(3):1917-32. doi: 10.1104/pp.15.00953. Epub 2015 Sep 10. Plant Physiol. 2015. PMID: 26358417 Free PMC article.
-
Exo70E2 is essential for exocyst subunit recruitment and EXPO formation in both plants and animals.Mol Biol Cell. 2014 Feb;25(3):412-26. doi: 10.1091/mbc.E13-10-0586. Epub 2013 Dec 4. Mol Biol Cell. 2014. PMID: 24307681 Free PMC article.
-
Arabinogalactan glycosyltransferases target to a unique subcellular compartment that may function in unconventional secretion in plants.Traffic. 2014 Nov;15(11):1219-34. doi: 10.1111/tra.12203. Epub 2014 Sep 12. Traffic. 2014. PMID: 25074762 Free PMC article.
-
Unconventional protein secretion in plants: a critical assessment.Protoplasma. 2016 Jan;253(1):31-43. doi: 10.1007/s00709-015-0887-1. Epub 2015 Sep 26. Protoplasma. 2016. PMID: 26410830 Review.
-
Exocyst complexes multiple functions in plant cells secretory pathways.Curr Opin Plant Biol. 2013 Dec;16(6):726-33. doi: 10.1016/j.pbi.2013.10.013. Epub 2013 Nov 15. Curr Opin Plant Biol. 2013. PMID: 24246229 Review.
Cited by
-
Genetic Screening of Factors in the Plant Protein Secretion.Methods Mol Biol. 2024;2841:225-239. doi: 10.1007/978-1-0716-4059-3_22. Methods Mol Biol. 2024. PMID: 39115782
-
The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment.Front Immunol. 2022 Jun 10;13:896745. doi: 10.3389/fimmu.2022.896745. eCollection 2022. Front Immunol. 2022. PMID: 35757759 Free PMC article. Review.
-
The trafficking of the cellulose synthase complex in higher plants.Ann Bot. 2014 Oct;114(6):1059-67. doi: 10.1093/aob/mcu040. Epub 2014 Mar 20. Ann Bot. 2014. PMID: 24651373 Free PMC article. Review.
-
Isolation and Characterization of Extracellular Vesicles from Arabidopsis thaliana Cell Culture and Investigation of the Specificities of Their Biogenesis.Plants (Basel). 2023 Oct 18;12(20):3604. doi: 10.3390/plants12203604. Plants (Basel). 2023. PMID: 37896067 Free PMC article.
-
Vesicle trafficking in rice: too little is known.Front Plant Sci. 2023 Sep 18;14:1263966. doi: 10.3389/fpls.2023.1263966. eCollection 2023. Front Plant Sci. 2023. PMID: 37790794 Free PMC article. Review.
References
-
- Baba M., Osumi M., Ohsumi Y. (1995). Analysis of the membrane structures involved in autophagy in yeast by freeze-replica method. Cell Struct. Funct. 20: 465–471 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

