Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species
- PMID: 21193400
- PMCID: PMC3060482
- DOI: 10.1074/jbc.M110.200501
Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species
Abstract
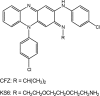
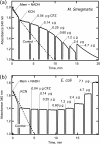
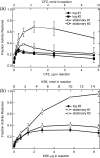
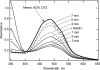
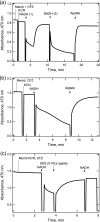
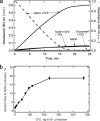
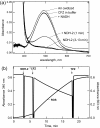
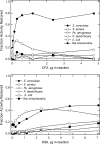
The mechanism of action of clofazimine (CFZ), an antimycobacterial drug with a long history, is not well understood. The present study describes a redox cycling pathway that involves the enzymatic reduction of CFZ by NDH-2, the primary respiratory chain NADH:quinone oxidoreductase of mycobacteria and nonenzymatic oxidation of reduced CFZ by O(2) yielding CFZ and reactive oxygen species (ROS). This pathway was demonstrated using isolated membranes and purified recombinant NDH-2. The reduction and oxidation of CFZ was measured spectrally, and the production of ROS was measured using a coupled assay system with Amplex Red. Supporting the ROS-based killing mechanism, bacteria grown in the presence of antioxidants are more resistant to CFZ. CFZ-mediated increase in NADH oxidation and ROS production were not observed in membranes from three different Gram-negative bacteria but was observed in Staphylococcus aureus and Saccharomyces cerevisiae, which is consistent with the known antimicrobial specificity of CFZ. A more soluble analog of CFZ, KS6, was synthesized and was shown to have the same activities as CFZ. These studies describe a pathway for a continuous and high rate of reactive oxygen species production in Mycobacterium smegmatis treated with CFZ and a CFZ analog as well as evidence that cell death produced by these agents are related to the production of these radical species.
Figures


Similar articles
-
Activation of type II NADH dehydrogenase by quinolinequinones mediates antitubercular cell death.J Antimicrob Chemother. 2016 Oct;71(10):2840-7. doi: 10.1093/jac/dkw244. Epub 2016 Jun 30. J Antimicrob Chemother. 2016. PMID: 27365187
-
The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress.Sci Rep. 2015 May 27;5:10333. doi: 10.1038/srep10333. Sci Rep. 2015. PMID: 26015371 Free PMC article.
-
Regulation of the mechanism of Type-II NADH: Quinone oxidoreductase from S. aureus.Redox Biol. 2018 Jun;16:209-214. doi: 10.1016/j.redox.2018.02.004. Epub 2018 Feb 17. Redox Biol. 2018. PMID: 29524843 Free PMC article.
-
Clofazimine: A useful antibiotic for drug-resistant tuberculosis.Biomed Pharmacother. 2018 Sep;105:1353-1359. doi: 10.1016/j.biopha.2018.06.023. Epub 2018 Jun 29. Biomed Pharmacother. 2018. PMID: 30021373 Review.
-
Clofazimine susceptibility testing of Mycobacterium avium complex and Mycobacterium abscessus: a meta-analysis study.J Glob Antimicrob Resist. 2021 Sep;26:188-193. doi: 10.1016/j.jgar.2021.06.002. Epub 2021 Jun 19. J Glob Antimicrob Resist. 2021. PMID: 34153525 Review.
Cited by
-
Persistent bacterial infections, antibiotic tolerance, and the oxidative stress response.Virulence. 2013 May 15;4(4):273-83. doi: 10.4161/viru.23987. Epub 2013 Apr 5. Virulence. 2013. PMID: 23563389 Free PMC article. Review.
-
Multiplexed transcriptional repression identifies a network of bactericidal interactions between mycobacterial respiratory complexes.iScience. 2021 Dec 4;25(1):103573. doi: 10.1016/j.isci.2021.103573. eCollection 2022 Jan 21. iScience. 2021. PMID: 34984329 Free PMC article.
-
Antitubercular drugs: possible role of natural products acting as antituberculosis medication in overcoming drug resistance and drug-induced hepatotoxicity.Naunyn Schmiedebergs Arch Pharmacol. 2024 Mar;397(3):1251-1273. doi: 10.1007/s00210-023-02679-z. Epub 2023 Sep 4. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 37665346 Review.
-
A cell-based high-throughput screen identifies drugs that cause bleeding disorders by off-targeting the vitamin K cycle.Blood. 2020 Aug 13;136(7):898-908. doi: 10.1182/blood.2019004234. Blood. 2020. PMID: 32374827 Free PMC article.
-
The Transcription Factor Rv1453 Regulates the Expression of qor and Confers Resistant to Clofazimine in Mycobacterium tuberculosis.Infect Drug Resist. 2021 Sep 24;14:3937-3948. doi: 10.2147/IDR.S324043. eCollection 2021. Infect Drug Resist. 2021. PMID: 34594117 Free PMC article.
References
-
- Barry V. C., Belton J. G., Conalty M. L., Denneny J. M., Edward D. W., O'Sullivan J. F., Twomey D., Winder F. (1957) Nature 179, 1013–1015 - PubMed
-
- O'Connor R., O'Sullivan J. F., O'Kennedy R. (1995) Drug Metab. Rev. 27, 591–614 - PubMed
-
- Reddy V. M., O'Sullivan J. F., Gangadharam P. R. J. (1999) J. Antimicrob. Chemother. 43, 615–623 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

