Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation
- PMID: 21190999
- PMCID: PMC3062411
- DOI: 10.1182/blood-2010-11-321208
Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation
Abstract
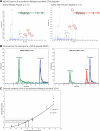
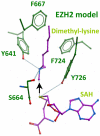
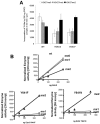
Next-generation sequencing of follicular lymphoma and diffuse-large B-cell lymphoma has revealed frequent somatic, heterozygous Y641 mutations in the histone methyltransferase EZH2. Heterozygosity and the presence of equal quantities of both mutant and wild-type mRNA and expressed protein suggest a dominant mode of action. Surprisingly, B-cell lymphoma cell lines and lymphoma samples harboring heterozygous EZH2(Y641) mutations have increased levels of histone H3 Lys-27-specific trimethylation (H3K27me3). Expression of EZH2(Y641F/N) mutants in cells with EZH2(WT) resulted in an increase of H3K27me3 levels in vivo. Structural modeling of EZH2(Y641) mutants suggests a "Tyr/Phe switch" model whereby structurally neutral, nontyrosine residues at position 641 would decrease affinity for unmethylated and monomethylated H3K27 substrates and potentially favor trimethylation. We demonstrate, using in vitro enzyme assays of reconstituted PRC2 complexes, that Y641 mutations result in a decrease in monomethylation and an increase in trimethylation activity of the enzyme relative to the wild-type enzyme. This represents the first example of a disease-associated gain-of-function mutation in a histone methyltransferase, whereby somatic EZH2 Y641 mutations in lymphoma act dominantly to increase, rather than decrease, histone methylation. The dominant mode of action suggests that allele-specific EZH2 inhibitors should be a future therapeutic strategy for this disease.
Figures

Similar articles
-
Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27).Proc Natl Acad Sci U S A. 2012 Feb 21;109(8):2989-94. doi: 10.1073/pnas.1116418109. Epub 2012 Feb 8. Proc Natl Acad Sci U S A. 2012. PMID: 22323599 Free PMC article.
-
EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations.Nature. 2012 Dec 6;492(7427):108-12. doi: 10.1038/nature11606. Epub 2012 Oct 10. Nature. 2012. PMID: 23051747
-
Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas.Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):20980-5. doi: 10.1073/pnas.1012525107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078963 Free PMC article.
-
Aberrations of EZH2 in cancer.Clin Cancer Res. 2011 May 1;17(9):2613-8. doi: 10.1158/1078-0432.CCR-10-2156. Epub 2011 Mar 2. Clin Cancer Res. 2011. PMID: 21367748 Review.
-
Targeting EZH2 and PRC2 dependence as novel anticancer therapy.Exp Hematol. 2015 Aug;43(8):698-712. doi: 10.1016/j.exphem.2015.05.001. Epub 2015 May 28. Exp Hematol. 2015. PMID: 26027790 Free PMC article. Review.
Cited by
-
Epimutational profile of hematologic malignancies as attractive target for new epigenetic therapies.Oncotarget. 2016 Aug 30;7(35):57327-57350. doi: 10.18632/oncotarget.10033. Oncotarget. 2016. PMID: 27329599 Free PMC article. Review.
-
Determinants of Chromatin Organization in Aging and Cancer-Emerging Opportunities for Epigenetic Therapies and AI Technology.Genes (Basel). 2024 May 29;15(6):710. doi: 10.3390/genes15060710. Genes (Basel). 2024. PMID: 38927646 Free PMC article. Review.
-
Parallel functional annotation of cancer-associated missense mutations in histone methyltransferases.Sci Rep. 2022 Nov 2;12(1):18487. doi: 10.1038/s41598-022-23229-2. Sci Rep. 2022. PMID: 36323913 Free PMC article.
-
H3K27 Methylation: A Focal Point of Epigenetic Deregulation in Cancer.Adv Cancer Res. 2016;131:59-95. doi: 10.1016/bs.acr.2016.05.001. Epub 2016 Jun 17. Adv Cancer Res. 2016. PMID: 27451124 Free PMC article. Review.
-
Poorly differentiated synovial sarcoma is associated with high expression of enhancer of zeste homologue 2 (EZH2).J Transl Med. 2012 Oct 30;10:216. doi: 10.1186/1479-5876-10-216. J Transl Med. 2012. PMID: 23110793 Free PMC article.
References
-
- Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

