Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma
- PMID: 21189380
- PMCID: PMC3646322
- DOI: 10.1200/JCO.2010.32.0770
Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma
Abstract
Purpose: To evaluate the safety and efficacy of a modified administration schedule of docetaxel, cisplatin, and fluorouracil (mDCF) with bevacizumab in patients with advanced gastroesophageal malignancies.
Patients and methods: Previously untreated patients with metastatic gastroesophageal adenocarcinoma received bevacizumab 10 mg/kg, docetaxel 40 mg/m², fluorouracil 400 mg/m², leucovorin 400 mg/m² on day 1, fluorouracil 1,000 mg/m²/d × 2 days intravenous continuous infusion beginning on day 1, and cisplatin 40 mg/m² on day 3. The primary objective was to improve 6-month progression-free survival (PFS) from 43% (historical DCF control) to 63% with the addition of bevacizumab. The target accrual was 44 patients to have 10% type I and II error rates.
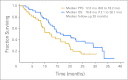
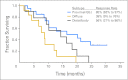
Results: In total, 44 eligible patients with cancer were enrolled from October 2006 to October 2008: 22 gastric, 20 gastroesophageal junction (GEJ), and two esophagus. In 39 patients with measurable disease, the confirmed response rate was 67% (95% CI, 50% to 81%). Six-month PFS was 79% (95% CI, 63% to 88%), and median PFS was 12 months (95% CI, 8.8 to 18.2 months). With 26-month follow-up, median overall survival (OS) was 16.8 months (95% CI, 12.1 to 26.1 months), and 2-year survival was 37%. Treatment-related grade 3 to 4 toxicity was as follows: neutropenia without fever (50%), fatigue (25%), venous thromboembolism (39%), and nausea, vomiting, mucositis, neuropathy, and febrile neutropenia less than 10% each. In subset analysis, diffuse gastric cancer had significantly worse PFS and OS, and the response rate in proximal/GEJ tumors was 85% (95% CI, 62% to 97%).
Conclusion: mDCF with bevacizumab appears tolerable and has notable patient outcomes in patients with advanced gastroesophageal adenocarcinoma. Six-month PFS was 79%, surpassing our predefined efficacy end point, and median and 2-year OS were 16.8 months and 37%, respectively.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Randomized Multicenter Phase II Study of Modified Docetaxel, Cisplatin, and Fluorouracil (DCF) Versus DCF Plus Growth Factor Support in Patients With Metastatic Gastric Adenocarcinoma: A Study of the US Gastric Cancer Consortium.J Clin Oncol. 2015 Nov 20;33(33):3874-9. doi: 10.1200/JCO.2015.60.7465. J Clin Oncol. 2015. PMID: 26438119 Clinical Trial.
-
Preoperative docetaxel/cisplatin/5-fluorouracil chemotherapy in patients with locally advanced gastro-esophageal adenocarcinoma.Med Oncol. 2012 Sep;29(3):1707-10. doi: 10.1007/s12032-011-0093-8. Epub 2011 Oct 28. Med Oncol. 2012. PMID: 22033912
-
Docetaxel, Oxaliplatin, and 5-Fluorouracil (DOF) in Metastatic and Unresectable Gastric/Gastroesophageal Junction Adenocarcinoma: A Phase II Study with Long-Term Follow-Up.Oncologist. 2019 Aug;24(8):1039-e642. doi: 10.1634/theoncologist.2019-0330. Epub 2019 May 28. Oncologist. 2019. PMID: 31138725 Free PMC article. Clinical Trial.
-
Docetaxel, cisplatin and fluorouracil (DCF) regimen compared with non-taxane-containing palliative chemotherapy for gastric carcinoma: a systematic review and meta-analysis.PLoS One. 2013 Apr 4;8(4):e60320. doi: 10.1371/journal.pone.0060320. Print 2013. PLoS One. 2013. PMID: 23593191 Free PMC article. Review.
-
FLOT or CROSS for gastroesophageal junction cancers-is the debate over yet?Chin Clin Oncol. 2023 Jun;12(3):24. doi: 10.21037/cco-23-5. Epub 2023 May 25. Chin Clin Oncol. 2023. PMID: 37303220 Review.
Cited by
-
Advances in targeted therapies and new promising targets in esophageal cancer.Oncotarget. 2015 Jan 30;6(3):1348-58. doi: 10.18632/oncotarget.2752. Oncotarget. 2015. PMID: 25593196 Free PMC article. Review.
-
Clinical implications of DLL4 expression in gastric cancer.J Exp Clin Cancer Res. 2013 Jul 30;32(1):46. doi: 10.1186/1756-9966-32-46. J Exp Clin Cancer Res. 2013. PMID: 23898884 Free PMC article.
-
Serum VEGF-A and Tumor Vessel VEGFR-2 Levels Predict Survival in Caucasian but Not Asian Patients Undergoing Resection for Gastric Adenocarcinoma.Ann Surg Oncol. 2015 Dec;22 Suppl 3(Suppl 3):S1508-15. doi: 10.1245/s10434-015-4790-y. Epub 2015 Aug 11. Ann Surg Oncol. 2015. PMID: 26259755 Free PMC article.
-
Panitumumab in combination with modified docetaxel/cisplatin/5-fluorouracil as first-line treatment in gastric and gastroesophageal junction adenocarcinomas: a multicenter phase II study by the Hellenic Oncology Research Group.Ann Gastroenterol. 2018 Nov-Dec;31(6):698-704. doi: 10.20524/aog.2018.0311. Epub 2018 Sep 14. Ann Gastroenterol. 2018. PMID: 30386120 Free PMC article.
-
Targeted therapies in gastric cancer and future perspectives.World J Gastroenterol. 2016 Jan 14;22(2):471-89. doi: 10.3748/wjg.v22.i2.471. World J Gastroenterol. 2016. PMID: 26811601 Free PMC article. Review.
References
-
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. - PubMed
-
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–2909. - PubMed
-
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. - PubMed
-
- Ajani JA, Moiseyenko VM, Tjulandin S, et al. Quality of life with docetaxel plus cisplatin and fluorouracil compared with cisplatin and fluorouracil from a phase III trial for advanced gastric or gastroesophageal adenocarcinoma: The V-325 Study Group. J Clin Oncol. 2007;25:3210–3216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

