NleC, a type III secretion protease, compromises NF-κB activation by targeting p65/RelA
- PMID: 21187904
- PMCID: PMC3002990
- DOI: 10.1371/journal.ppat.1001231
NleC, a type III secretion protease, compromises NF-κB activation by targeting p65/RelA
Abstract
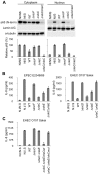
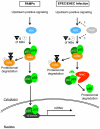
The NF-κB signaling pathway is central to the innate and adaptive immune responses. Upon their detection of pathogen-associated molecular patterns, Toll-like receptors on the cell surface initiate signal transduction and activate the NF-κB pathway, leading to the production of a wide array of inflammatory cytokines, in attempt to eradicate the invaders. As a countermeasure, pathogens have evolved ways to subvert and manipulate this system to their advantage. Enteropathogenic and enterohemorrhagic Escherichia coli (EPEC and EHEC) are closely related bacteria responsible for major food-borne diseases worldwide. Via a needle-like protein complex called the type three secretion system (T3SS), these pathogens deliver virulence factors directly to host cells and modify cellular functions, including by suppressing the inflammatory response. Using gain- and loss-of-function screenings, we identified two bacterial effectors, NleC and NleE, that down-regulate the NF-κB signal upon being injected into a host cell via the T3SS. A recent report showed that NleE inhibits NF-κB activation, although an NleE-deficient pathogen was still immune-suppressive, indicating that other anti-inflammatory effectors are involved. In agreement, our present results showed that NleC was also required to inhibit inflammation. We found that NleC is a zinc protease that disrupts NF-κB activation by the direct cleavage of NF-κB's p65 subunit in the cytoplasm, thereby decreasing the available p65 and reducing the total nuclear entry of active p65. More importantly, we showed that a mutant EPEC/EHEC lacking both NleC and NleE (ΔnleC ΔnleE) caused greater inflammatory response than bacteria carrying ΔnleC or ΔnleE alone. This effect was similar to that of a T3SS-defective mutant. In conclusion, we found that NleC is an anti-inflammatory bacterial zinc protease, and that the cooperative function of NleE and NleC disrupts the NF-κB pathway and accounts for most of the immune suppression caused by EHEC/EPEC.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
A type III effector protease NleC from enteropathogenic Escherichia coli targets NF-κB for degradation.Mol Microbiol. 2011 Apr;80(1):219-30. doi: 10.1111/j.1365-2958.2011.07568.x. Epub 2011 Feb 22. Mol Microbiol. 2011. PMID: 21306441 Free PMC article.
-
T3SS-Independent Uptake of the Short-Trip Toxin-Related Recombinant NleC Effector of Enteropathogenic Escherichia coli Leads to NF-κB p65 Cleavage.Front Cell Infect Microbiol. 2017 Apr 13;7:119. doi: 10.3389/fcimb.2017.00119. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28451521 Free PMC article.
-
Metalloprotease NleC suppresses host NF-κB/inflammatory responses by cleaving p65 and interfering with the p65/RPS3 interaction.PLoS Pathog. 2015 Mar 10;11(3):e1004705. doi: 10.1371/journal.ppat.1004705. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25756944 Free PMC article.
-
Modulation of the Inflammasome Signaling Pathway by Enteropathogenic and Enterohemorrhagic Escherichia coli.Front Cell Infect Microbiol. 2016 Aug 26;6:89. doi: 10.3389/fcimb.2016.00089. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27617233 Free PMC article. Review.
-
Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements.Mol Microbiol. 2011 Jun;80(6):1420-38. doi: 10.1111/j.1365-2958.2011.07661.x. Epub 2011 May 5. Mol Microbiol. 2011. PMID: 21488979 Review.
Cited by
-
The enteropathogenic E. coli (EPEC) Tir effector inhibits NF-κB activity by targeting TNFα receptor-associated factors.PLoS Pathog. 2011 Dec;7(12):e1002414. doi: 10.1371/journal.ppat.1002414. Epub 2011 Dec 1. PLoS Pathog. 2011. PMID: 22144899 Free PMC article.
-
NleB2 from enteropathogenic Escherichia coli is a novel arginine-glucose transferase effector.PLoS Pathog. 2021 Jun 16;17(6):e1009658. doi: 10.1371/journal.ppat.1009658. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34133469 Free PMC article.
-
Microbial strategies for antagonizing Toll-like-receptor signal transduction.Curr Opin Immunol. 2015 Feb;32:61-70. doi: 10.1016/j.coi.2014.12.011. Epub 2015 Jan 20. Curr Opin Immunol. 2015. PMID: 25615700 Free PMC article. Review.
-
Cyclic dinucleotides and the innate immune response.Cell. 2013 Aug 29;154(5):962-970. doi: 10.1016/j.cell.2013.08.014. Cell. 2013. PMID: 23993090 Free PMC article. Review.
-
Proteolytic Cleavage of NF-κB p65: A Novel Mechanism for Subversion of Innate Immune Signaling by Pathogenic E. Coli.Front Microbiol. 2011 Mar 1;2:38. doi: 10.3389/fmicb.2011.00038. eCollection 2011. Front Microbiol. 2011. PMID: 21833300 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

