The intracellular dynamic of protein palmitoylation
- PMID: 21187327
- PMCID: PMC3010063
- DOI: 10.1083/jcb.201008160
The intracellular dynamic of protein palmitoylation
Abstract
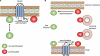
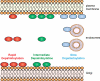
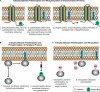
S-palmitoylation describes the reversible attachment of fatty acids (predominantly palmitate) onto cysteine residues via a labile thioester bond. This posttranslational modification impacts protein functionality by regulating membrane interactions, intracellular sorting, stability, and membrane micropatterning. Several recent findings have provided a tantalizing insight into the regulation and spatiotemporal dynamics of protein palmitoylation. In mammalian cells, the Golgi has emerged as a possible super-reaction center for the palmitoylation of peripheral membrane proteins, whereas palmitoylation reactions on post-Golgi compartments contribute to the regulation of specific substrates. In addition to palmitoylating and depalmitoylating enzymes, intracellular palmitoylation dynamics may also be controlled through interplay with distinct posttranslational modifications, such as phosphorylation and nitrosylation.
Figures
Similar articles
-
S-Palmitoylation of a Novel Site in the β2-Adrenergic Receptor Associated with a Novel Intracellular Itinerary.J Biol Chem. 2016 Sep 16;291(38):20232-46. doi: 10.1074/jbc.M116.725762. Epub 2016 Aug 1. J Biol Chem. 2016. PMID: 27481942 Free PMC article.
-
Protein palmitoylation and its pathophysiological relevance.J Cell Physiol. 2021 May;236(5):3220-3233. doi: 10.1002/jcp.30122. Epub 2020 Oct 22. J Cell Physiol. 2021. PMID: 33094504 Review.
-
Palmitoylation and the trafficking of peripheral membrane proteins.Biochem Soc Trans. 2013 Feb 1;41(1):62-6. doi: 10.1042/BST20120243. Biochem Soc Trans. 2013. PMID: 23356259
-
Stress-induced Changes in the S-palmitoylation and S-nitrosylation of Synaptic Proteins.Mol Cell Proteomics. 2019 Oct;18(10):1916-1938. doi: 10.1074/mcp.RA119.001581. Epub 2019 Jul 16. Mol Cell Proteomics. 2019. PMID: 31311849 Free PMC article.
-
Palmitoylation mechanisms in dopamine transporter regulation.J Chem Neuroanat. 2017 Oct;83-84:3-9. doi: 10.1016/j.jchemneu.2017.01.002. Epub 2017 Jan 20. J Chem Neuroanat. 2017. PMID: 28115272 Free PMC article. Review.
Cited by
-
Cysteine 70 of ankyrin-G is S-palmitoylated and is required for function of ankyrin-G in membrane domain assembly.J Biol Chem. 2012 Dec 21;287(52):43995-4005. doi: 10.1074/jbc.M112.417501. Epub 2012 Nov 5. J Biol Chem. 2012. PMID: 23129772 Free PMC article.
-
H-ras distribution and signaling in plasma membrane microdomains are regulated by acylation and deacylation events.Mol Cell Biol. 2015 Jun 1;35(11):1898-914. doi: 10.1128/MCB.01398-14. Epub 2015 Mar 16. Mol Cell Biol. 2015. PMID: 25776558 Free PMC article.
-
Palmitoylation controls trafficking of the intracellular Ca2+ channel MCOLN3/TRPML3 to regulate autophagy.Autophagy. 2019 Feb;15(2):327-340. doi: 10.1080/15548627.2018.1518671. Epub 2018 Sep 14. Autophagy. 2019. PMID: 30215288 Free PMC article.
-
Exploring protein lipidation with chemical biology.Chem Rev. 2011 Oct 12;111(10):6341-58. doi: 10.1021/cr2001977. Epub 2011 Sep 16. Chem Rev. 2011. PMID: 21919527 Free PMC article. Review. No abstract available.
-
How prenylation and S-acylation regulate subcellular targeting and function of ROP GTPases.Plant Signal Behav. 2011 Jul;6(7):1026-9. doi: 10.4161/psb.6.7.15578. Plant Signal Behav. 2011. PMID: 21694496 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

