RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis
- PMID: 21173229
- PMCID: PMC3017198
- DOI: 10.1073/pnas.1017241108
RNA editing enzyme adenosine deaminase is a restriction factor for controlling measles virus replication that also is required for embryogenesis
Abstract
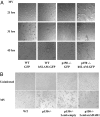
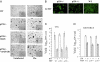
Measles virus (MV), a member of the family Paramyxoviridae and an exclusively human pathogen, is among the most infectious viruses. A progressive fatal neurodegenerative complication, subacute sclerosing panencephalitis (SSPE), occurs during persistent MV infection of the CNS and is associated with biased hypermutations of the viral genome. The observed hypermutations of A-to-G are consistent with conversions catalyzed by the adenosine deaminase acting on RNA (ADAR1). To evaluate the role of ADAR1 in MV infection, we selectively disrupted expression of the IFN-inducible p150 ADAR1 isoform and found it caused embryonic lethality at embryo day (E) 11-E12. We therefore generated p150-deficient and WT mouse embryo fibroblast (MEF) cells stably expressing the MV receptor signaling lymphocyte activation molecule (SLAM or CD150). The p150(-/-) but not WT MEF cells displayed extensive syncytium formation and cytopathic effect (CPE) following infection with MV, consistent with an anti-MV role of the p150 isoform of ADAR1. MV titers were 3 to 4 log higher in p150(-/-) cells compared with WT cells at 21 h postinfection, and restoration of ADAR1 in p150(-/-) cells prevented MV cytopathology. In contrast to infection with MV, p150 disruption had no effect on vesicular stomatitis virus, reovirus, or lymphocytic choriomeningitis virus replication but protected against CPE resulting from infection with Newcastle disease virus, Sendai virus, canine distemper virus, and influenza A virus. Thus, ADAR1 is a restriction factor in the replication of paramyxoviruses and orthomyxoviruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Is the p150 isoform of the RNA editing enzyme Adenosine Deaminase 1 really responsible for embryonic lethality?Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):E43; author reply E44. doi: 10.1073/pnas.1100419108. Epub 2011 Mar 14. Proc Natl Acad Sci U S A. 2011. PMID: 21402945 Free PMC article. No abstract available.
-
ADAR1 isoform involvement in embryonic lethality.Proc Natl Acad Sci U S A. 2011 Jun 14;108(24):E199; author reply E200. doi: 10.1073/pnas.1105004108. Epub 2011 May 18. Proc Natl Acad Sci U S A. 2011. PMID: 21593418 Free PMC article. No abstract available.
Similar articles
-
Is the p150 isoform of the RNA editing enzyme Adenosine Deaminase 1 really responsible for embryonic lethality?Proc Natl Acad Sci U S A. 2011 Mar 22;108(12):E43; author reply E44. doi: 10.1073/pnas.1100419108. Epub 2011 Mar 14. Proc Natl Acad Sci U S A. 2011. PMID: 21402945 Free PMC article. No abstract available.
-
Measles Virus Defective Interfering RNAs Are Generated Frequently and Early in the Absence of C Protein and Can Be Destabilized by Adenosine Deaminase Acting on RNA-1-Like Hypermutations.J Virol. 2015 Aug;89(15):7735-47. doi: 10.1128/JVI.01017-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972541 Free PMC article.
-
Receptor use by vesicular stomatitis virus pseudotypes with glycoproteins of defective variants of measles virus isolated from brains of patients with subacute sclerosing panencephalitis.J Gen Virol. 2003 Aug;84(Pt 8):2133-2143. doi: 10.1099/vir.0.19091-0. J Gen Virol. 2003. PMID: 12867645
-
Modeling subacute sclerosing panencephalitis in a transgenic mouse system: uncoding pathogenesis of disease and illuminating components of immune control.Curr Top Microbiol Immunol. 2009;330:31-54. doi: 10.1007/978-3-540-70617-5_2. Curr Top Microbiol Immunol. 2009. PMID: 19203103 Review.
-
Deciphering the Biological Significance of ADAR1-Z-RNA Interactions.Int J Mol Sci. 2021 Oct 23;22(21):11435. doi: 10.3390/ijms222111435. Int J Mol Sci. 2021. PMID: 34768866 Free PMC article. Review.
Cited by
-
Multi-level regulation of cellular recognition of viral dsRNA.Cell Mol Life Sci. 2013 Jun;70(11):1949-63. doi: 10.1007/s00018-012-1149-4. Epub 2012 Sep 9. Cell Mol Life Sci. 2013. PMID: 22960755 Free PMC article. Review.
-
Influenza virus partially counteracts restriction imposed by tetherin/BST-2.J Biol Chem. 2012 Jun 22;287(26):22015-29. doi: 10.1074/jbc.M111.319996. Epub 2012 Apr 6. J Biol Chem. 2012. PMID: 22493439 Free PMC article.
-
The role of RNA editing enzyme ADAR1 in human disease.Wiley Interdiscip Rev RNA. 2022 Jan;13(1):e1665. doi: 10.1002/wrna.1665. Epub 2021 Jun 8. Wiley Interdiscip Rev RNA. 2022. PMID: 34105255 Free PMC article. Review.
-
Malignant A-to-I RNA editing by ADAR1 drives T cell acute lymphoblastic leukemia relapse via attenuating dsRNA sensing.Cell Rep. 2024 Feb 27;43(2):113704. doi: 10.1016/j.celrep.2024.113704. Epub 2024 Jan 23. Cell Rep. 2024. PMID: 38265938 Free PMC article.
-
Measles virus, immune control, and persistence.FEMS Microbiol Rev. 2012 May;36(3):649-62. doi: 10.1111/j.1574-6976.2012.00330.x. Epub 2012 Mar 13. FEMS Microbiol Rev. 2012. PMID: 22316382 Free PMC article. Review.
References
-
- Griffin DE. Measles virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 1551–1585.
-
- Oldstone MB. Modeling subacute sclerosing panencephalitis in a transgenic mouse system: Uncoding pathogenesis of disease and illuminating components of immune control. Curr Top Microbiol Immunol. 2009;330:31–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

