Rab27a is required for human cytomegalovirus assembly
- PMID: 21170347
- PMCID: PMC2999566
- DOI: 10.1371/journal.pone.0015318
Rab27a is required for human cytomegalovirus assembly
Abstract
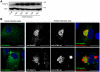
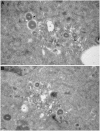
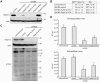
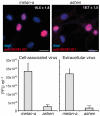
Human cytomegalovirus (HCMV) completes its final envelopment on intracellular membranes before it is released from the cell. The mechanisms underlying these processes are not understood. Here we studied the role of Rab27a, a regulator of lysosome-related organelle transport, in HCMV production. HCMV infection increased Rab27a expression, and recruitment of Rab27a to membranous strutures at the assembly site. Immuno-gold labelling demonstrated association of Rab27a with viral envelopes. CMV production was reduced after knock-down of Rab27a, and in Rab27a-deficient ashen melanocytes. This study shows a requirement for Rab27a in the CMV life cycle and suggests that CMV and LRO biogenesis share common molecular mechanisms.
Conflict of interest statement
Figures

Similar articles
-
Rab27a enables myosin Va-dependent melanosome capture by recruiting the myosin to the organelle.J Cell Sci. 2001 Mar;114(Pt 6):1091-100. doi: 10.1242/jcs.114.6.1091. J Cell Sci. 2001. PMID: 11228153
-
The leaden gene product is required with Rab27a to recruit myosin Va to melanosomes in melanocytes.Traffic. 2002 Mar;3(3):193-202. doi: 10.1034/j.1600-0854.2002.030305.x. Traffic. 2002. PMID: 11886590
-
Rab3GEP is the non-redundant guanine nucleotide exchange factor for Rab27a in melanocytes.J Biol Chem. 2008 Aug 22;283(34):23209-16. doi: 10.1074/jbc.M804134200. Epub 2008 Jun 17. J Biol Chem. 2008. PMID: 18559336 Free PMC article.
-
Rab GTPases, intracellular traffic and disease.Trends Mol Med. 2002 Jan;8(1):23-30. doi: 10.1016/s1471-4914(01)02227-4. Trends Mol Med. 2002. PMID: 11796263 Review.
-
Role of Rab family GTPases and their effectors in melanosomal logistics.J Biochem. 2012 Apr;151(4):343-51. doi: 10.1093/jb/mvs009. Epub 2012 Feb 9. J Biochem. 2012. PMID: 22323658 Review.
Cited by
-
A Portrait of the Human Organelle Proteome In Space and Time during Cytomegalovirus Infection.Cell Syst. 2016 Oct 26;3(4):361-373.e6. doi: 10.1016/j.cels.2016.08.012. Epub 2016 Sep 15. Cell Syst. 2016. PMID: 27641956 Free PMC article.
-
Human cytomegalovirus-infected cells release extracellular vesicles that carry viral surface proteins.Virology. 2018 Nov;524:97-105. doi: 10.1016/j.virol.2018.08.008. Epub 2018 Aug 27. Virology. 2018. PMID: 30165311 Free PMC article.
-
Exosome Biogenesis, Regulation, and Function in Viral Infection.Viruses. 2015 Sep 17;7(9):5066-83. doi: 10.3390/v7092862. Viruses. 2015. PMID: 26393640 Free PMC article. Review.
-
Monoubiquitination of syntaxin 3 leads to retrieval from the basolateral plasma membrane and facilitates cargo recruitment to exosomes.Mol Biol Cell. 2017 Oct 15;28(21):2843-2853. doi: 10.1091/mbc.E17-07-0461. Epub 2017 Aug 16. Mol Biol Cell. 2017. PMID: 28814500 Free PMC article.
-
Extracellular Vesicles in Viral Spread and Antiviral Response.Viruses. 2020 Jun 8;12(6):623. doi: 10.3390/v12060623. Viruses. 2020. PMID: 32521696 Free PMC article. Review.
References
-
- Mocarski ES, Shenk T, Pass RF. Cytomegaloviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology, 5th ed. Philadelphia, Pa.: Lippincott-Raven Publishers; 2007. pp. 2701–2772.
-
- Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143:222–234. - PubMed
-
- Fraile-Ramos A, Pelchen-Matthews A, Kledal TN, Browne H, Schwartz TW, et al. Localization of HCMV UL33 and US27 in Endocytic Compartments and Viral Membranes. Traffic. 2002;3:218–232. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

