Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission
- PMID: 21170049
- PMCID: PMC3059246
- DOI: 10.1038/nsmb.1949
Conformational changes in Dnm1 support a contractile mechanism for mitochondrial fission
Abstract
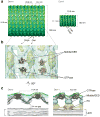
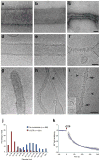
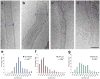
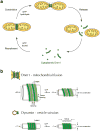
Mitochondria are dynamic organelles that undergo cycles of fission and fusion. The yeast dynamin-related protein Dnm1 has been localized to sites of mitochondrial division. Using cryo-EM, we have determined the three-dimensional (3D) structure of Dnm1 in a GTP-bound state. The 3D map showed that Dnm1 adopted a unique helical assembly when compared with dynamin, which is involved in vesicle scission during endocytosis. Upon GTP hydrolysis, Dnm1 constricted liposomes and subsequently dissociated from the lipid bilayer. The magnitude of Dnm1 constriction was substantially larger than the decrease in diameter previously reported for dynamin. We postulate that the larger conformational change is mediated by a flexible Dnm1 structure that has limited interaction with the underlying bilayer. Our structural studies support the idea that Dnm1 has a mechanochemical role during mitochondrial division.
Conflict of interest statement
Figures

Similar articles
-
Interchangeable adaptors regulate mitochondrial dynamin assembly for membrane scission.Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):E1342-51. doi: 10.1073/pnas.1300855110. Epub 2013 Mar 25. Proc Natl Acad Sci U S A. 2013. PMID: 23530241 Free PMC article.
-
Molecular architecture of a dynamin adaptor: implications for assembly of mitochondrial fission complexes.J Cell Biol. 2010 Dec 13;191(6):1127-39. doi: 10.1083/jcb.201005046. J Cell Biol. 2010. PMID: 21149566 Free PMC article.
-
The mechanoenzymatic core of dynamin-related protein 1 comprises the minimal machinery required for membrane constriction.J Biol Chem. 2015 May 1;290(18):11692-703. doi: 10.1074/jbc.M114.610881. Epub 2015 Mar 13. J Biol Chem. 2015. PMID: 25770210 Free PMC article.
-
Molecular Basis of Mitochondrial and Peroxisomal Division Machineries.Int J Mol Sci. 2020 Jul 30;21(15):5452. doi: 10.3390/ijms21155452. Int J Mol Sci. 2020. PMID: 32751702 Free PMC article. Review.
-
Dynamin: functional design of a membrane fission catalyst.Annu Rev Cell Dev Biol. 2011;27:79-105. doi: 10.1146/annurev-cellbio-100109-104016. Epub 2011 May 18. Annu Rev Cell Dev Biol. 2011. PMID: 21599493 Review.
Cited by
-
Mitochondrial Quality Control Orchestrates the Symphony of B Cells and Plays Critical Roles in B Cell-Related Diseases.J Immunol Res. 2024 Oct 17;2024:5577506. doi: 10.1155/2024/5577506. eCollection 2024. J Immunol Res. 2024. PMID: 39449998 Free PMC article. Review.
-
Molecular mechanisms of mitochondrial dynamics.Nat Rev Mol Cell Biol. 2024 Oct 17. doi: 10.1038/s41580-024-00785-1. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39420231 Review.
-
SUMOylation of MFF coordinates fission complexes to promote stress-induced mitochondrial fragmentation.Sci Adv. 2024 Oct 4;10(40):eadq6223. doi: 10.1126/sciadv.adq6223. Epub 2024 Oct 4. Sci Adv. 2024. PMID: 39365854 Free PMC article.
-
Dynamins combine mechano-constriction and membrane remodeling to enable two-step mitochondrial fission via a 'snap-through' instability.bioRxiv [Preprint]. 2024 Aug 20:2024.08.19.608723. doi: 10.1101/2024.08.19.608723. bioRxiv. 2024. PMID: 39229060 Free PMC article. Preprint.
-
Atg44/Mdi1/mitofissin facilitates Dnm1-mediated mitochondrial fission.Autophagy. 2024 Oct;20(10):2314-2322. doi: 10.1080/15548627.2024.2360345. Epub 2024 Jun 4. Autophagy. 2024. PMID: 38818923 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

