The binary switch that controls the life and death decisions of ER stressed β cells
- PMID: 21168319
- PMCID: PMC3078183
- DOI: 10.1016/j.ceb.2010.11.005
The binary switch that controls the life and death decisions of ER stressed β cells
Abstract
Diabetes mellitus is a group of common metabolic disorders defined by hyperglycemia. One of the most important factors contributing to hyperglycemia is dysfunction and death of β cells. Increasing experimental, clinical, and genetic evidence indicates that endoplasmic reticulum (ER) stress plays an important role in β cell dysfunction and death during the progression of type 1 and type 2 diabetes as well as genetic forms of diabetes such as Wolfram syndrome. The mechanisms of ER stress-mediated β cell dysfunction and death are complex and not homogenous. Here we review the recent key findings on the role of ER stress and the unfolded protein response (UPR) in β cells and the mechanisms of ER stress-mediated β cell dysfunction and death. Complete understanding of these mechanisms will lead to novel therapeutic modalities for diabetes.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
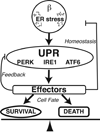
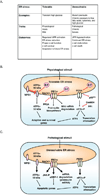
There are two types of ER stress conditions: tolerable and unresolvable. Tolerable ER stress can be physiological, acute, and/or mild. Under tolerable ER stress conditions, the UPR is properly activated and regulated reducing ER stress and promoting β cell adaptation and survival. In contrast, unresolvable ER stress conditions are pathological, chronic, and/or severe. Under unresolvable ER stress conditions, the UPR is hyperactivated inducing β cell dysfunction and death.
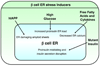
When β cells are exposed to physiological stimuli that induces tolerable ER stress, the UPR triggers transcription of adaptive and survival genes, attenuates translation, and promotes mild mRNA degradation to reduce ER stress. The UPR also promotes proinsulin biosynthesis. As ER homeostasis is restablished, BiP, Gadd34, P58IPK, RACK1 and WFS1 properly turn off different components of the UPR.
When β cells are under pathological conditions that induce unresolvable ER stress, the UPR is hyperactivated triggering the activation of apoptotic pathways and upregulating apoptotic genes. The UPR also mediates degradation of mRNAs encoding proinsulin and ER homeostatic proteins. Under these conditions, the UPR bypasses feedback regulation.
Similar articles
-
Endoplasmic reticulum stress and pancreatic β-cell death.Trends Endocrinol Metab. 2011 Jul;22(7):266-74. doi: 10.1016/j.tem.2011.02.008. Epub 2011 Mar 31. Trends Endocrinol Metab. 2011. PMID: 21458293 Free PMC article. Review.
-
The binary switch between life and death of endoplasmic reticulum-stressed beta cells.Curr Opin Endocrinol Diabetes Obes. 2010 Apr;17(2):107-12. doi: 10.1097/MED.0b013e3283372843. Curr Opin Endocrinol Diabetes Obes. 2010. PMID: 20125004 Free PMC article. Review.
-
Endoplasmic reticulum stress in beta-cells and development of diabetes.Curr Opin Pharmacol. 2009 Dec;9(6):763-70. doi: 10.1016/j.coph.2009.07.003. Epub 2009 Aug 6. Curr Opin Pharmacol. 2009. PMID: 19665428 Free PMC article. Review.
-
Stress hypERactivation in the β-cell.Islets. 2010 Jan-Feb;2(1):1-9. doi: 10.4161/isl.2.1.10456. Islets. 2010. PMID: 21099287 Review.
-
Endoplasmic reticulum stress, degeneration of pancreatic islet β-cells, and therapeutic modulation of the unfolded protein response in diabetes.Mol Metab. 2019 Sep;27S(Suppl):S60-S68. doi: 10.1016/j.molmet.2019.06.012. Mol Metab. 2019. PMID: 31500832 Free PMC article. Review.
Cited by
-
ER stress as a trigger for β-cell dysfunction and autoimmunity in type 1 diabetes.Diabetes. 2012 Apr;61(4):780-1. doi: 10.2337/db12-0091. Diabetes. 2012. PMID: 22442299 Free PMC article. No abstract available.
-
Differential ER stress as a driver of cell fate following ricin toxin exposure.FASEB Bioadv. 2021 Oct 19;4(1):60-75. doi: 10.1096/fba.2021-00005. eCollection 2022 Jan. FASEB Bioadv. 2021. PMID: 35024573 Free PMC article.
-
Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress.Cold Spring Harb Perspect Biol. 2018 Jul 2;10(7):a032870. doi: 10.1101/cshperspect.a032870. Cold Spring Harb Perspect Biol. 2018. PMID: 29440070 Free PMC article. Review.
-
Layer 2/3 pyramidal cells in the medial prefrontal cortex moderate stress induced depressive behaviors.Elife. 2015 Sep 15;4:e08752. doi: 10.7554/eLife.08752. Elife. 2015. PMID: 26371510 Free PMC article.
-
The Role of Damage-Associated Molecular Patterns (DAMPs) in Human Diseases: Part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine.Sultan Qaboos Univ Med J. 2015 May;15(2):e157-70. Epub 2015 May 28. Sultan Qaboos Univ Med J. 2015. PMID: 26052447 Free PMC article. Review.
References
-
- Donath MY, Halban PA. Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia. 2004;47:581–589. - PubMed
-
-
Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. References and propose that β cell death is a critical pathogenic component in both type 1 and type 2 diabetes.
-
-
-
Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 2010 This is the first article proposing that the UPR acts as a bianary switch regulating life and death of ER stressed β cells.
-
-
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. - PubMed
-
-
Fonseca SG, Ishigaki S, Oslowski CM, Lu S, Lipson KL, Ghosh R, Hayashi E, Ishihara H, Oka Y, Permutt MA, et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest. 2010 This article reports that β cell death in Wolfram syndrome is caused by the hyperactivation of ER stress signaling.
-
Publication types
MeSH terms
Grants and funding
- R01 DK067493-04/DK/NIDDK NIH HHS/United States
- R01 DK067493-07/DK/NIDDK NIH HHS/United States
- R01 DK067493-02/DK/NIDDK NIH HHS/United States
- P30 DK032520/DK/NIDDK NIH HHS/United States
- R01 DK067493-03/DK/NIDDK NIH HHS/United States
- 5 P30 DK32520/DK/NIDDK NIH HHS/United States
- R01 DK067493-04S1/DK/NIDDK NIH HHS/United States
- R01 DK067493/DK/NIDDK NIH HHS/United States
- R01DK067493/DK/NIDDK NIH HHS/United States
- R01 DK067493-05/DK/NIDDK NIH HHS/United States
- R01 DK067493-06A1/DK/NIDDK NIH HHS/United States
- R01 DK067493-01A2/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

