Cooperative epigenetic modulation by cancer amplicon genes
- PMID: 21156283
- PMCID: PMC3049192
- DOI: 10.1016/j.ccr.2010.11.013
Cooperative epigenetic modulation by cancer amplicon genes
Abstract
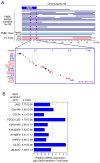
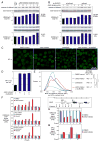
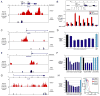
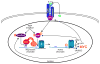
Chromosome band 9p24 is frequently amplified in primary mediastinal B cell lymphoma (PMBL) and Hodgkin lymphoma (HL). To identify oncogenes in this amplicon, we screened an RNA interference library targeting amplicon genes and thereby identified JAK2 and the histone demethylase JMJD2C as essential genes in these lymphomas. Inhibition of JAK2 and JMJD2C cooperated in killing these lymphomas by decreasing tyrosine 41 phosphorylation and increasing lysine 9 trimethylation of histone H3, promoting heterochromatin formation. MYC, a major target of JAK2-mediated histone phosphorylation, was silenced after JAK2 and JMJD2C inhibition, with a corresponding increase in repressive chromatin. Hence, JAK2 and JMJD2C cooperatively remodel the PMBL and HL epigenome, offering a mechanistic rationale for the development of JAK2 and JMJD2C inhibitors in these diseases.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Partners in crime: Genes within an amplicon collude to globally deregulate chromatin in lymphoma.Cancer Cell. 2010 Dec 14;18(6):539-41. doi: 10.1016/j.ccr.2010.11.032. Cancer Cell. 2010. PMID: 21156276 Free PMC article.
Similar articles
-
Biallelic mutation of SOCS-1 impairs JAK2 degradation and sustains phospho-JAK2 action in the MedB-1 mediastinal lymphoma line.Blood. 2005 Mar 15;105(6):2535-42. doi: 10.1182/blood-2004-09-3701. Epub 2004 Nov 30. Blood. 2005. PMID: 15572583
-
Report: workshop on mediastinal grey zone lymphoma.Eur J Haematol Suppl. 2005 Jul;(66):45-52. doi: 10.1111/j.1600-0609.2005.00454.x. Eur J Haematol Suppl. 2005. PMID: 16007868
-
Dynamic mathematical modeling of IL13-induced signaling in Hodgkin and primary mediastinal B-cell lymphoma allows prediction of therapeutic targets.Cancer Res. 2011 Feb 1;71(3):693-704. doi: 10.1158/0008-5472.CAN-10-2987. Epub 2010 Dec 2. Cancer Res. 2011. PMID: 21127196
-
KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells.Cancer Res. 2013 May 15;73(10):2936-42. doi: 10.1158/0008-5472.CAN-12-4300. Epub 2013 May 3. Cancer Res. 2013. PMID: 23644528 Free PMC article. Review.
-
Histone demethylase JMJD2C: epigenetic regulators in tumors.Oncotarget. 2017 Jul 12;8(53):91723-91733. doi: 10.18632/oncotarget.19176. eCollection 2017 Oct 31. Oncotarget. 2017. PMID: 29207681 Free PMC article. Review.
Cited by
-
Roles of histone methyl-modifying enzymes in development and progression of cancer.Cancer Sci. 2013 Jul;104(7):795-800. doi: 10.1111/cas.12169. Epub 2013 May 9. Cancer Sci. 2013. PMID: 23560485 Free PMC article. Review.
-
Immune escape mechanisms for TCRLBCL.Blood. 2021 Mar 11;137(10):1274-1276. doi: 10.1182/blood.2020008766. Blood. 2021. PMID: 33704396 Free PMC article.
-
KDM4 Demethylases: Structure, Function, and Inhibitors.Adv Exp Med Biol. 2023;1433:87-111. doi: 10.1007/978-3-031-38176-8_5. Adv Exp Med Biol. 2023. PMID: 37751137
-
The JmjN domain as a dimerization interface and a targeted inhibitor of KDM4 demethylase activity.Oncotarget. 2018 Mar 30;9(24):16861-16882. doi: 10.18632/oncotarget.24717. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682190 Free PMC article.
-
Diffuse large B-cell lymphoma-treatment approaches in the molecular era.Nat Rev Clin Oncol. 2014 Jan;11(1):12-23. doi: 10.1038/nrclinonc.2013.197. Epub 2013 Nov 12. Nat Rev Clin Oncol. 2014. PMID: 24217204 Free PMC article. Review.
References
-
- Andrews AL, Holloway JW, Puddicombe SM, Holgate ST, Davies DE. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073–46078. - PubMed
-
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410:120–124. - PubMed
-
- Bentz M, Barth TF, Bruderlein S, Bock D, Schwerer MJ, Baudis M, Joos S, Viardot A, Feller AC, Muller-Hermelink HK, et al. Gain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell line. Genes Chromosomes Cancer. 2001;30:393–401. - PubMed
-
- Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. - PubMed
-
- Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

