Multiplexing of multicolor bioluminescence resonance energy transfer
- PMID: 21156147
- PMCID: PMC3000516
- DOI: 10.1016/j.bpj.2010.10.025
Multiplexing of multicolor bioluminescence resonance energy transfer
Abstract
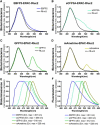
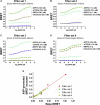
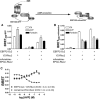
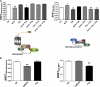
Bioluminescence resonance energy transfer (BRET) is increasingly being used to monitor protein-protein interactions and cellular events in cells. However, the ability to monitor multiple events simultaneously is limited by the spectral properties of the existing BRET partners. Taking advantage of newly developed Renilla luciferases and blue-shifted fluorescent proteins (FPs), we explored the possibility of creating novel BRET configurations using a single luciferase substrate and distinct FPs. Three new (to our knowledge) BRET assays leading to distinct color bioluminescence emission were generated and validated. The spectral properties of two of the FPs used (enhanced blue (EB) FP2 and mAmetrine) and the selection of appropriate detection filters permitted the concomitant detection of two independent BRET signals, without cross-interference, in the same cells after addition of a unique substrate for Renilla luciferase-II, coelentrazine-400a. Using individual BRET-based biosensors to monitor the interaction between G-protein-coupled receptors and G-protein subunits or activation of different G-proteins along with the production of a second messenger, we established the proof of principle that two new BRET configurations can be multiplexed to simultaneously monitor two dependent or independent cellular events. The development of this new multiplexed BRET configuration opens the way for concomitant monitoring of various independent biological processes in living cells.
Copyright © 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Real-time analysis of agonist-induced activation of protease-activated receptor 1/Galphai1 protein complex measured by bioluminescence resonance energy transfer in living cells.Mol Pharmacol. 2007 May;71(5):1329-40. doi: 10.1124/mol.106.030304. Epub 2007 Jan 31. Mol Pharmacol. 2007. PMID: 17267663
-
Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells.Methods Mol Biol. 2015;1278:457-65. doi: 10.1007/978-1-4939-2425-7_30. Methods Mol Biol. 2015. PMID: 25859969 Free PMC article.
-
Bioluminescence resonance energy transfer-based imaging of protein-protein interactions in living cells.Nat Protoc. 2019 Apr;14(4):1084-1107. doi: 10.1038/s41596-019-0129-7. Epub 2019 Mar 25. Nat Protoc. 2019. PMID: 30911173
-
Bioluminescence Resonance Energy Transfer as a Method to Study Protein-Protein Interactions: Application to G Protein Coupled Receptor Biology.Molecules. 2019 Feb 1;24(3):537. doi: 10.3390/molecules24030537. Molecules. 2019. PMID: 30717191 Free PMC article. Review.
-
Fluorescence/bioluminescence resonance energy transfer techniques to study G-protein-coupled receptor activation and signaling.Pharmacol Rev. 2012 Apr;64(2):299-336. doi: 10.1124/pr.110.004309. Epub 2012 Mar 8. Pharmacol Rev. 2012. PMID: 22407612 Review.
Cited by
-
The free energy landscape in translational science: how can somatic mutations result in constitutive oncogenic activation?Phys Chem Chem Phys. 2014 Apr 14;16(14):6332-41. doi: 10.1039/c3cp54253j. Epub 2014 Jan 21. Phys Chem Chem Phys. 2014. PMID: 24445437 Free PMC article.
-
Identification and characterization of an activating F229V substitution in the V2 vasopressin receptor in an infant with NSIAD.J Am Soc Nephrol. 2012 Oct;23(10):1635-40. doi: 10.1681/ASN.2012010077. Epub 2012 Sep 6. J Am Soc Nephrol. 2012. PMID: 22956819 Free PMC article.
-
Interphase microtubule disassembly is a signaling cue that drives cell rounding at mitotic entry.J Cell Biol. 2022 Jun 6;221(6):e202109065. doi: 10.1083/jcb.202109065. Epub 2022 Apr 28. J Cell Biol. 2022. PMID: 35482006 Free PMC article.
-
Bioluminescent Systems for Theranostic Applications.Int J Mol Sci. 2024 Jul 10;25(14):7563. doi: 10.3390/ijms25147563. Int J Mol Sci. 2024. PMID: 39062805 Free PMC article. Review.
-
A Perspective on Studying G-Protein-Coupled Receptor Signaling with Resonance Energy Transfer Biosensors in Living Organisms.Mol Pharmacol. 2015 Sep;88(3):589-95. doi: 10.1124/mol.115.098897. Epub 2015 May 13. Mol Pharmacol. 2015. PMID: 25972446 Free PMC article. Review.
References
-
- Morin J.G., Hastings J.W. Energy transfer in a bioluminescent system. J. Cell. Physiol. 1971;77:313–318. - PubMed
-
- Dacres H., Wang J., Trowell S.C. Experimental determination of the Förster distance for two commonly used bioluminescent resonance energy transfer pairs. Anal. Chem. 2010;82:432–435. - PubMed
-
- Pfleger K.D., Eidne K.A. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET) Nat. Methods. 2006;3:165–174. - PubMed
-
- Hamdan F.F., Percherancier Y., Bouvier M. MonitorinG-protein-protein interactions in living cells by bioluminescence resonance energy transfer (BRET) Curr. Protoc. Neurosci. 2006 Chapter 5:Unit 5.23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

