Dynamics of digestive proteolytic system during blood feeding of the hard tick Ixodes ricinus
- PMID: 21156061
- PMCID: PMC3016361
- DOI: 10.1186/1756-3305-3-119
Dynamics of digestive proteolytic system during blood feeding of the hard tick Ixodes ricinus
Abstract
Background: Ticks are vectors of a wide variety of pathogens causing severe diseases in humans and domestic animals. Intestinal digestion of the host blood is an essential process of tick physiology and also a limiting factor for pathogen transmission since the tick gut represents the primary site for pathogen infection and proliferation. Using the model tick Ixodes ricinus, the European Lyme disease vector, we have previously demonstrated by genetic and biochemical analyses that host blood is degraded in the tick gut by a network of acidic peptidases of the aspartic and cysteine classes.
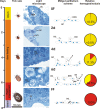
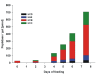
Results: This study reveals the digestive machinery of the I. ricinus during the course of blood-feeding on the host. The dynamic profiling of concentrations, activities and mRNA expressions of the major digestive enzymes demonstrates that the de novo synthesis of peptidases triggers the dramatic increase of the hemoglobinolytic activity along the feeding period. Overall hemoglobinolysis, as well as the activity of digestive peptidases are negligible at the early stage of feeding, but increase dramatically towards the end of the slow feeding period, reaching maxima in fully fed ticks. This finding contradicts the established opinion that blood digestion is reduced at the end of engorgement. Furthermore, we show that the digestive proteolysis is localized intracellularly throughout the whole duration of feeding.
Conclusions: Results suggest that the egressing proteolytic system in the early stage of feeding and digestion is a potential target for efficient impairment, most likely by blocking its components via antibodies present in the host blood. Therefore, digestive enzymes are promising candidates for development of novel 'anti-tick' vaccines capable of tick control and even transmission of tick-borne pathogens.
Figures
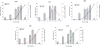

Similar articles
-
Profiling of proteolytic enzymes in the gut of the tick Ixodes ricinus reveals an evolutionarily conserved network of aspartic and cysteine peptidases.Parasit Vectors. 2008 Mar 18;1(1):7. doi: 10.1186/1756-3305-1-7. Parasit Vectors. 2008. PMID: 18348719 Free PMC article.
-
Multienzyme degradation of host serum albumin in ticks.Ticks Tick Borne Dis. 2016 Jun;7(4):604-13. doi: 10.1016/j.ttbdis.2015.12.014. Epub 2015 Dec 19. Ticks Tick Borne Dis. 2016. PMID: 26724897
-
Mialostatin, a Novel Midgut Cystatin from Ixodes ricinus Ticks: Crystal Structure and Regulation of Host Blood Digestion.Int J Mol Sci. 2021 May 20;22(10):5371. doi: 10.3390/ijms22105371. Int J Mol Sci. 2021. PMID: 34065290 Free PMC article.
-
A longitudinal transcriptomic analysis from unfed to post-engorgement midguts of adult female Ixodes scapularis.Sci Rep. 2023 Jul 13;13(1):11360. doi: 10.1038/s41598-023-38207-5. Sci Rep. 2023. PMID: 37443274 Free PMC article. Review.
-
Multi-trophic interactions driving the transmission cycle of Borrelia afzelii between Ixodes ricinus and rodents: a review.Parasit Vectors. 2015 Dec 18;8:643. doi: 10.1186/s13071-015-1257-8. Parasit Vectors. 2015. PMID: 26684199 Free PMC article. Review.
Cited by
-
Interactions between Borrelia burgdorferi and ticks.Nat Rev Microbiol. 2020 Oct;18(10):587-600. doi: 10.1038/s41579-020-0400-5. Epub 2020 Jul 10. Nat Rev Microbiol. 2020. PMID: 32651470 Free PMC article. Review.
-
High throughput techniques to reveal the molecular physiology and evolution of digestion in spiders.BMC Genomics. 2016 Sep 7;17(1):716. doi: 10.1186/s12864-016-3048-9. BMC Genomics. 2016. PMID: 27604083 Free PMC article.
-
A physiologic overview of the organ-specific transcriptome of the cattle tick Rhipicephalus microplus.Sci Rep. 2020 Oct 26;10(1):18296. doi: 10.1038/s41598-020-75341-w. Sci Rep. 2020. PMID: 33106528 Free PMC article.
-
Insight Into the Dynamics of the Ixodes ricinus Nymphal Midgut Proteome.Mol Cell Proteomics. 2023 Nov;22(11):100663. doi: 10.1016/j.mcpro.2023.100663. Epub 2023 Oct 12. Mol Cell Proteomics. 2023. PMID: 37832788 Free PMC article.
-
Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar).Vaccines (Basel). 2022 Jul 1;10(7):1063. doi: 10.3390/vaccines10071063. Vaccines (Basel). 2022. PMID: 35891227 Free PMC article.
References
-
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl):S3–14. - PubMed
-
- Sonenshine DE. Biology of ticks. New York: Oxford University Press; 1991.
-
- Nuttall PA. Pathogen-tick-host interactions: Borrelia burgdorferi and TBE virus. Zentralbl Bakteriol. 1999;289:492–505. - PubMed
LinkOut - more resources
Full Text Sources

