Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays
- PMID: 21152012
- PMCID: PMC2996325
- DOI: 10.1371/journal.ppat.1001217
Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays
Abstract
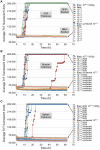
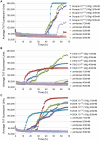
A major problem for the effective diagnosis and management of prion diseases is the lack of rapid high-throughput assays to measure low levels of prions. Such measurements have typically required prolonged bioassays in animals. Highly sensitive, but generally non-quantitative, prion detection methods have been developed based on prions' ability to seed the conversion of normally soluble protease-sensitive forms of prion protein to protease-resistant and/or amyloid fibrillar forms. Here we describe an approach for estimating the relative amount of prions using a new prion seeding assay called real-time quaking induced conversion assay (RT-QuIC). The underlying reaction blends aspects of the previously described quaking-induced conversion (QuIC) and amyloid seeding assay (ASA) methods and involves prion-seeded conversion of the alpha helix-rich form of bacterially expressed recombinant PrP(C) to a beta sheet-rich amyloid fibrillar form. The RT-QuIC is as sensitive as the animal bioassay, but can be accomplished in 2 days or less. Analogous to end-point dilution animal bioassays, this approach involves testing of serial dilutions of samples and statistically estimating the seeding dose (SD) giving positive responses in 50% of replicate reactions (SD(50)). Brain tissue from 263K scrapie-affected hamsters gave SD(50) values of 10(11)-10(12)/g, making the RT-QuIC similar in sensitivity to end-point dilution bioassays. Analysis of bioassay-positive nasal lavages from hamsters affected with transmissible mink encephalopathy gave SD(50) values of 10(3.5)-10(5.7)/ml, showing that nasal cavities release substantial prion infectivity that can be rapidly detected. Cerebral spinal fluid from 263K scrapie-affected hamsters contained prion SD(50) values of 10(2.0)-10(2.9)/ml. RT-QuIC assay also discriminated deer chronic wasting disease and sheep scrapie brain samples from normal control samples. In principle, end-point dilution quantitation can be applied to many types of prion and amyloid seeding assays. End point dilution RT-QuIC provides a sensitive, rapid, quantitative, and high throughput assay of prion seeding activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Prion seeding activities of mouse scrapie strains with divergent PrPSc protease sensitivities and amyloid plaque content using RT-QuIC and eQuIC.PLoS One. 2012;7(11):e48969. doi: 10.1371/journal.pone.0048969. Epub 2012 Nov 5. PLoS One. 2012. PMID: 23139828 Free PMC article.
-
Sensitive and specific detection of classical scrapie prions in the brains of goats by real-time quaking-induced conversion.J Gen Virol. 2016 Mar;97(3):803-812. doi: 10.1099/jgv.0.000367. Epub 2015 Dec 10. J Gen Virol. 2016. PMID: 26653410 Free PMC article.
-
Factors That Improve RT-QuIC Detection of Prion Seeding Activity.Viruses. 2016 May 23;8(5):140. doi: 10.3390/v8050140. Viruses. 2016. PMID: 27223300 Free PMC article.
-
New generation QuIC assays for prion seeding activity.Prion. 2012 Apr-Jun;6(2):147-52. doi: 10.4161/pri.19430. Epub 2012 Apr 1. Prion. 2012. PMID: 22421206 Free PMC article. Review.
-
RT-QuIC Assays in Humans … and Animals.Food Saf (Tokyo). 2016 Dec 7;4(4):115-120. doi: 10.14252/foodsafetyfscj.2016020. eCollection 2016 Dec. Food Saf (Tokyo). 2016. PMID: 32231915 Free PMC article. Review.
Cited by
-
Accelerated shedding of prions following damage to the olfactory epithelium.J Virol. 2012 Feb;86(3):1777-88. doi: 10.1128/JVI.06626-11. Epub 2011 Nov 30. J Virol. 2012. PMID: 22130543 Free PMC article.
-
Different post-mortem brain regions from three Chinese FFI patients induce different reactive profiles both in the first and second generation RT-QuIC assays.Prion. 2020 Dec;14(1):163-169. doi: 10.1080/19336896.2020.1782809. Prion. 2020. PMID: 32573356 Free PMC article.
-
The Latest Research on RT-QuIC Assays-A Literature Review.Pathogens. 2021 Mar 5;10(3):305. doi: 10.3390/pathogens10030305. Pathogens. 2021. PMID: 33807776 Free PMC article. Review.
-
Protein misfolding detected early in pathogenesis of transgenic mouse model of Huntington disease using amyloid seeding assay.J Biol Chem. 2012 Mar 23;287(13):9982-9989. doi: 10.1074/jbc.M111.305417. Epub 2011 Dec 20. J Biol Chem. 2012. PMID: 22187438 Free PMC article.
-
Prion protein-Semisynthetic prion protein (PrP) variants with posttranslational modifications.J Pept Sci. 2019 Oct;25(10):e3216. doi: 10.1002/psc.3216. J Pept Sci. 2019. PMID: 31713950 Free PMC article. Review.
References
-
- Caughey B, Raymond GJ. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991;266:18217–18223. - PubMed
-
- Castilla J, Saa P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

