A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients
- PMID: 21151619
- PMCID: PMC2998804
- DOI: 10.2147/DDDT.S14071
A Phase I study of an intravesically administered immunotoxin targeting EpCAM for the treatment of nonmuscle-invasive bladder cancer in BCGrefractory and BCG-intolerant patients
Abstract
Purpose: A Phase I dose-escalation study was performed to determine the maximum tolerated dose (MTD) of the immunotoxin VB4-845 in patients with nonmuscle-invasive bladder cancer (NMIBC) refractory to or intolerant of bacillus Calmette-Guerin (BCG). Secondary objectives included evaluation of the safety, tolerability, pharmacokinetics, immunogenicity, and efficacy of VB4-845.
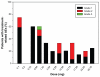
Patients and methods: Sixty-four patients with Grade 2 or 3, stage Ta or T1 transitional cell carcinoma or in situ carcinoma, either refractory to or intolerant of BCG therapy, were enrolled. Treatment was administered in ascending dose cohorts ranging from 0.1 to 30.16 mg. After receiving weekly instillations of VB4-845 to the bladder via catheter for 6 consecutive weeks, patients were followed for 4-6 weeks post-therapy and assessed at week 12.
Results: An MTD was not determined, as a dose-limiting toxicity was not identified over the dose range tested. VB4-845 therapy was safe and well tolerated with most adverse events reported as mild; as a result, no patients were removed from the study in response to toxicity. By the end of the study, the majority of patients had developed antibodies to the exotoxin portion of VB4-845. A complete response was achieved in 39% of patients at the 12-week time point.
Conclusions: VB4-845 dosed on a weekly basis for 6 weeks was very well tolerated at all dose levels. Although an MTD was not determined at the doses administered, VB4-845 showed evidence of an antitumor effect that warrants further clinical investigation for the treatment of NMIBC in this patient population.
Keywords: Pseudomonas exotoxin A; anti-EpCAM; fusion protein; targeted therapy.
Figures

Similar articles
-
The effect of age on the efficacy of maintenance bacillus Calmette-Guérin relative to maintenance epirubicin in patients with stage Ta T1 urothelial bladder cancer: results from EORTC genito-urinary group study 30911.Eur Urol. 2014 Oct;66(4):694-701. doi: 10.1016/j.eururo.2014.05.033. Epub 2014 Jun 16. Eur Urol. 2014. PMID: 24948466 Clinical Trial.
-
A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin.J Urol. 2012 Nov;188(5):1712-8. doi: 10.1016/j.juro.2012.07.020. Epub 2012 Sep 19. J Urol. 2012. PMID: 22998907 Clinical Trial.
-
Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guérin immunotherapy.J Urol. 2013 Oct;190(4):1192-9. doi: 10.1016/j.juro.2013.04.077. Epub 2013 May 3. J Urol. 2013. PMID: 23648222
-
Management of high-risk non-muscle invasive bladder cancer.Minerva Urol Nefrol. 2012 Dec;64(4):255-60. Minerva Urol Nefrol. 2012. PMID: 23288212 Review.
-
Diagnosis and management of superficial bladder cancer.Curr Probl Cancer. 2001 Jul-Aug;25(4):219-78. doi: 10.1067/mcn.2001.117539. Curr Probl Cancer. 2001. PMID: 11514784 Review.
Cited by
-
Advances in anticancer immunotoxin therapy.Oncologist. 2015 Feb;20(2):176-85. doi: 10.1634/theoncologist.2014-0358. Epub 2015 Jan 5. Oncologist. 2015. PMID: 25561510 Free PMC article. Review.
-
The expansion of targetable biomarkers for CAR T cell therapy.J Exp Clin Cancer Res. 2018 Jul 21;37(1):163. doi: 10.1186/s13046-018-0817-0. J Exp Clin Cancer Res. 2018. PMID: 30031396 Free PMC article. Review.
-
A bispecific EpCAM/CD133-targeted toxin is effective against carcinoma.Target Oncol. 2014 Sep;9(3):239-49. doi: 10.1007/s11523-013-0290-9. Epub 2013 Jul 31. Target Oncol. 2014. PMID: 23900680 Free PMC article.
-
From Interferon to Checkpoint Inhibition Therapy-A Systematic Review of New Immune-Modulating Agents in Bacillus Calmette-Guérin (BCG) Refractory Non-Muscle-Invasive Bladder Cancer (NMIBC).Cancers (Basel). 2022 Jan 29;14(3):694. doi: 10.3390/cancers14030694. Cancers (Basel). 2022. PMID: 35158964 Free PMC article. Review.
-
Novel intravesical therapeutics in the treatment of non-muscle invasive bladder cancer: Horizon scanning.Front Surg. 2022 Jul 26;9:912438. doi: 10.3389/fsurg.2022.912438. eCollection 2022. Front Surg. 2022. PMID: 35959122 Free PMC article. Review.
References
-
- Bladder Cancer Version 1. Jenkintown (PA): National Comprehensive Cancer Network (NCCN); 2005.
- Clinical Practice Guidelines in Oncology, Version 1. 2008. [Accessed 2009 Nov 4]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- Shah SC, Kusiak A, O’Donnell MA. Patient-recognition data-mining model for BCG-plus interferon immunotherapy bladder cancer treatment. Comput Biol Med. 2006;36(6):634–655. - PubMed
-
- Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical Bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized trials. J Urol. 2002;168(5):1964–1970. - PubMed
-
- Sylvester RJ, van der Meijden A, Witjes JA, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66(6 Suppl 1):90–107. - PubMed
-
- Boyd LA. Intravesical Bacillus Calmette-Guerin for treating bladder cancer. Urol Nurs. 2003;23(3):189–191. 199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

