The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner
- PMID: 21148306
- PMCID: PMC3059047
- DOI: 10.1074/jbc.M110.203638
The BH3 alpha-helical mimic BH3-M6 disrupts Bcl-X(L), Bcl-2, and MCL-1 protein-protein interactions with Bax, Bak, Bad, or Bim and induces apoptosis in a Bax- and Bim-dependent manner
Abstract
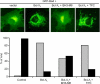
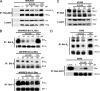
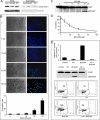
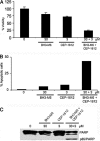
A critical hallmark of cancer cell survival is evasion of apoptosis. This is commonly due to overexpression of anti-apoptotic proteins such as Bcl-2, Bcl-X(L), and Mcl-1, which bind to the BH3 α-helical domain of pro-apoptotic proteins such as Bax, Bak, Bad, and Bim, and inhibit their function. We designed a BH3 α-helical mimetic BH3-M6 that binds to Bcl-X(L) and Mcl-1 and prevents their binding to fluorescently labeled Bak- or Bim-BH3 peptides in vitro. Using several approaches, we demonstrate that BH3-M6 is a pan-Bcl-2 antagonist that inhibits the binding of Bcl-X(L), Bcl-2, and Mcl-1 to multi-domain Bax or Bak, or BH3-only Bim or Bad in cell-free systems and in intact human cancer cells, freeing up pro-apoptotic proteins to induce apoptosis. BH3-M6 disruption of these protein-protein interactions is associated with cytochrome c release from mitochondria, caspase-3 activation and PARP cleavage. Using caspase inhibitors and Bax and Bak siRNAs, we demonstrate that BH3-M6-induced apoptosis is caspase- and Bax-, but not Bak-dependent. Furthermore, BH3-M6 disrupts Bcl-X(L)/Bim, Bcl-2/Bim, and Mcl-1/Bim protein-protein interactions and frees up Bim to induce apoptosis in human cancer cells that depend for tumor survival on the neutralization of Bim with Bcl-X(L), Bcl-2, or Mcl-1. Finally, BH3-M6 sensitizes cells to apoptosis induced by the proteasome inhibitor CEP-1612.
Figures



Similar articles
-
BH3 domains other than Bim and Bid can directly activate Bax/Bak.J Biol Chem. 2011 Jan 7;286(1):491-501. doi: 10.1074/jbc.M110.167148. Epub 2010 Nov 1. J Biol Chem. 2011. PMID: 21041309 Free PMC article.
-
Bax/Bak activation in the absence of Bid, Bim, Puma, and p53.Cell Death Dis. 2016 Jun 16;7(6):e2266. doi: 10.1038/cddis.2016.167. Cell Death Dis. 2016. PMID: 27310874 Free PMC article.
-
Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies.Nat Cell Biol. 2006 Dec;8(12):1348-58. doi: 10.1038/ncb1499. Epub 2006 Nov 19. Nat Cell Biol. 2006. PMID: 17115033
-
Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels.Cell Cycle. 2007 Sep 15;6(18):2236-40. doi: 10.4161/cc.6.18.4728. Epub 2007 Jul 10. Cell Cycle. 2007. PMID: 17881896 Review.
-
Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review.
Cited by
-
Selective and potent proteomimetic inhibitors of intracellular protein-protein interactions.Angew Chem Int Ed Engl. 2015 Mar 2;54(10):2960-5. doi: 10.1002/anie.201410810. Epub 2015 Feb 4. Angew Chem Int Ed Engl. 2015. PMID: 25651514 Free PMC article.
-
DARC: Mapping Surface Topography by Ray-Casting for Effective Virtual Screening at Protein Interaction Sites.J Med Chem. 2016 May 12;59(9):4152-70. doi: 10.1021/acs.jmedchem.5b00150. Epub 2015 Jul 10. J Med Chem. 2016. PMID: 26126123 Free PMC article.
-
Discovery of PI-1840, a novel noncovalent and rapidly reversible proteasome inhibitor with anti-tumor activity.J Biol Chem. 2014 Apr 25;289(17):11906-11915. doi: 10.1074/jbc.M113.533950. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24570003 Free PMC article.
-
Cotargeting BCL-2 and PI3K Induces BAX-Dependent Mitochondrial Apoptosis in AML Cells.Cancer Res. 2018 Jun 1;78(11):3075-3086. doi: 10.1158/0008-5472.CAN-17-3024. Epub 2018 Mar 20. Cancer Res. 2018. PMID: 29559471 Free PMC article.
-
Cooperative effect of BI-69A11 and celecoxib enhances radiosensitization by modulating DNA damage repair in colon carcinoma.Tumour Biol. 2016 May;37(5):6389-402. doi: 10.1007/s13277-015-4399-6. Epub 2015 Dec 2. Tumour Biol. 2016. PMID: 26631035
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

