Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection
- PMID: 21147915
- PMCID: PMC3028904
- DOI: 10.1128/JVI.01967-10
Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection
Abstract
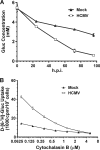
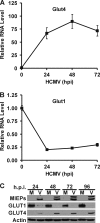
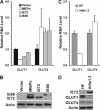
Glucose transport into mammalian cells is mediated by a group of glucose transporters (GLUTs) on the plasma membrane. Human cytomegalovirus (HCMV)-infected human fibroblasts (HFs) demonstrate significantly increased glucose consumption compared to mock-infected cells, suggesting a possible alteration in glucose transport during infection. Inhibition of GLUTs by using cytochalasin B indicated that infected cells utilize GLUT4, whereas normal HFs use GLUT1. Quantitative reverse transcription-PCR and Western analysis confirmed that GLUT4 levels are greatly increased in infected cells. In contrast, GLUT1 was eliminated by a mechanism involving the HCMV major immediate-early protein IE72. The HCMV-mediated induction of GLUT4 circumvents characterized controls of GLUT4 expression that involve serum stimulation, glucose concentration, and nuclear functions of ATP-citrate lyase (ACL). In infected cells the well-characterized Akt-mediated translocation of GLUT4 to the cell surface is also circumvented; GLUT4 localized on the surface of infected cells that were serum starved and had Akt activity inhibited. The significance of GLUT4 induction for the success of HCMV infection was indicated using indinavir, a drug that specifically inhibits glucose uptake by GLUT4. The addition of the drug inhibited glucose uptake in infected cells as well as viral production. Our data show that HCMV-specific mechanisms are used to replace GLUT1, the normal HF GLUT, with GLUT4, the major glucose transporter in adipose tissue, which has a 3-fold-higher glucose transport capacity.
Figures
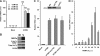

Similar articles
-
Beta3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation.Endocrinology. 2006 Dec;147(12):5730-9. doi: 10.1210/en.2006-0242. Epub 2006 Sep 7. Endocrinology. 2006. PMID: 16959848
-
Regulation of myocardial glucose transporters GLUT1 and GLUT4 in chronically anemic fetal lambs.Pediatr Res. 2005 Oct;58(4):713-8. doi: 10.1203/01.PDR.0000180546.42475.69. Pediatr Res. 2005. PMID: 16189198
-
Intracellular organization of insulin signaling and GLUT4 translocation.Recent Prog Horm Res. 2001;56:175-93. doi: 10.1210/rp.56.1.175. Recent Prog Horm Res. 2001. PMID: 11237212 Review.
-
Insulin sensitivity and inhibition by forskolin, dipyridamole and pentobarbital of glucose transport in three L6 muscle cell lines.Sci China C Life Sci. 2007 Dec;50(6):739-47. doi: 10.1007/s11427-007-0088-z. Epub 2007 Sep 20. Sci China C Life Sci. 2007. PMID: 17882384
-
Regulation of glucose transport by hypoxia.Am J Kidney Dis. 1999 Jul;34(1):189-202. doi: 10.1016/s0272-6386(99)70131-9. Am J Kidney Dis. 1999. PMID: 10401038 Review.
Cited by
-
A putative cell surface receptor for white spot syndrome virus is a member of a transporter superfamily.PLoS One. 2012;7(3):e33216. doi: 10.1371/journal.pone.0033216. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22427993 Free PMC article.
-
Identification of Selective Inhibitors of the Plasmodium falciparum Hexose Transporter PfHT by Screening Focused Libraries of Anti-Malarial Compounds.PLoS One. 2015 Apr 20;10(4):e0123598. doi: 10.1371/journal.pone.0123598. eCollection 2015. PLoS One. 2015. PMID: 25894322 Free PMC article.
-
Targeted Metabolic Reprogramming to Improve the Efficacy of Oncolytic Virus Therapy.Mol Ther. 2020 Jun 3;28(6):1417-1421. doi: 10.1016/j.ymthe.2020.03.014. Epub 2020 Mar 20. Mol Ther. 2020. PMID: 32243836 Free PMC article. Review.
-
Meal for Two: Human Cytomegalovirus-Induced Activation of Cellular Metabolism.Viruses. 2019 Mar 19;11(3):273. doi: 10.3390/v11030273. Viruses. 2019. PMID: 30893762 Free PMC article. Review.
-
Human herpesvirus 6A promotes glycolysis in infected T cells by activation of mTOR signaling.PLoS Pathog. 2020 Jun 9;16(6):e1008568. doi: 10.1371/journal.ppat.1008568. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32516328 Free PMC article.
References
-
- Al-Hasani, H., D. R. Yver, and S. W. Cushman. 1999. Overexpression of the glucose transporter GLUT4 in adipose cells interferes with insulin-stimulated translocation. FEBS Lett. 460:338-342. - PubMed
-
- Arnoni, C. P., et al. 2009. Regulation of glucose uptake in mesangial cells stimulated by high glucose: role of angiotensin II and insulin. Exp. Biol. Med. (Maywood) 234:1095-1101. - PubMed
-
- Bardell, D. 1984. Host cell glucose metabolism during abortive infection by adenovirus type 12. Microbios 39:95-99. - PubMed
-
- Bilan, P. J., Y. Mitsumoto, T. Ramlal, and A. Klip. 1992. Acute and long-term effects of insulin-like growth factor I on glucose transporters in muscle cells. Translocation and biosynthesis. FEBS Lett. 298:285-290. - PubMed
-
- Birnbaum, M. J. 1989. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell 57:305-315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

