Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence
- PMID: 21147463
- PMCID: PMC3013631
- DOI: 10.1016/j.chom.2010.11.005
Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence
Abstract
Macrophages are specialized to detect and destroy intracellular microbes and yet a number of pathogens have evolved to exploit this hostile niche. Here we demonstrate that the obligate intracellular parasite Toxoplasma gondii disarms macrophage innate clearance mechanisms by secreting a serine threonine kinase called ROP18, which binds to and phosphorylates immunity-related GTPases (IRGs). Substrate profiling of ROP18 revealed a preference for a conserved motif within switch region I of the GTPase domain, a modification predicted to disrupt IRG function. Consistent with this, expression of ROP18 was both necessary and sufficient to block recruitment of Irgb6, which was in turn required for parasite destruction. ROP18 phosphorylation of IRGs prevented clearance within inflammatory monocytes and IFN-γ-activated macrophages, conferring parasite survival in vivo and promoting virulence. IRGs are implicated in clearance of a variety of intracellular pathogens, suggesting that other virulence factors may similarly thwart this innate cellular defense mechanism.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


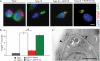
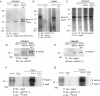

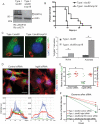
Comment in
-
Parasites paralyze cellular host defense system to promote virulence.Cell Host Microbe. 2010 Dec 16;8(6):463-4. doi: 10.1016/j.chom.2010.11.012. Cell Host Microbe. 2010. PMID: 21147459 Free PMC article.
Similar articles
-
Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):1126-31. doi: 10.1073/pnas.1313501111. Epub 2014 Jan 3. Proc Natl Acad Sci U S A. 2014. PMID: 24390541 Free PMC article.
-
Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii.PLoS Biol. 2010 Dec 21;8(12):e1000576. doi: 10.1371/journal.pbio.1000576. PLoS Biol. 2010. PMID: 21203588 Free PMC article.
-
The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18.PLoS Pathog. 2012;8(11):e1002992. doi: 10.1371/journal.ppat.1002992. Epub 2012 Nov 8. PLoS Pathog. 2012. PMID: 23144612 Free PMC article.
-
The secreted kinase ROP18 defends Toxoplasma's border.Bioessays. 2011 Sep;33(9):693-700. doi: 10.1002/bies.201100054. Epub 2011 Jul 20. Bioessays. 2011. PMID: 21773979 Free PMC article. Review.
-
The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii.Curr Opin Microbiol. 2011 Aug;14(4):414-21. doi: 10.1016/j.mib.2011.07.002. Epub 2011 Jul 23. Curr Opin Microbiol. 2011. PMID: 21783405 Review.
Cited by
-
iTRAQ-Based Phosphoproteomic Analysis Exposes Molecular Changes in the Small Intestinal Epithelia of Cats after Toxoplasma gondii Infection.Animals (Basel). 2023 Nov 16;13(22):3537. doi: 10.3390/ani13223537. Animals (Basel). 2023. PMID: 38003154 Free PMC article.
-
Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii.Parasitol Res. 2017 Jan;116(1):359-370. doi: 10.1007/s00436-016-5298-5. Epub 2016 Oct 27. Parasitol Res. 2017. PMID: 27785602
-
The Toxoplasma gondii rhoptry protein ROP18 is an Irga6-specific kinase and regulated by the dense granule protein GRA7.Cell Microbiol. 2016 Feb;18(2):244-59. doi: 10.1111/cmi.12499. Epub 2015 Oct 30. Cell Microbiol. 2016. PMID: 26247512 Free PMC article.
-
Reduced parasite motility and micronemal protein secretion by a p38 MAPK inhibitor leads to a severe impairment of cell invasion by the apicomplexan parasite Eimeria tenella.PLoS One. 2015 Feb 17;10(2):e0116509. doi: 10.1371/journal.pone.0116509. eCollection 2015. PLoS One. 2015. PMID: 25689363 Free PMC article.
-
Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins.PLoS One. 2012;7(7):e40391. doi: 10.1371/journal.pone.0040391. Epub 2012 Jul 12. PLoS One. 2012. PMID: 22808150 Free PMC article.
References
-
- Bastin P, Bagherzadeh Z, Matthews KR, Gull K. A novel epitope tag system to study protein targeting and organelle biogenesis in Trypanosoma brucei. Molec Biochem Parasitol. 1996;77:235–239. - PubMed
-
- Charif H, Darcy F, Torpier G, Cesbron-Delauw MF, Capron A. Toxoplasma gondii: characterization and localization of antigens secreted from tachyzoites. Exp Parasitol. 1990;71:114–124. - PubMed
-
- Coers J, Bernstein-Hanley I, Grotsky D, Parvanova I, Howard JC, Taylor GA, Dietrich WF, Starnbach MN. Chlamydia muridarum evades growth restriction by the IFN-gamma-inducible host resistance factor Irgb10. J Immunol. 2008;180:6237–6245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI084570/AI/NIAID NIH HHS/United States
- R01 AI082423/AI/NIAID NIH HHS/United States
- R01 AI036629/AI/NIAID NIH HHS/United States
- R21 AI091461/AI/NIAID NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- AI084243/AI/NIAID NIH HHS/United States
- R01 AI082423-02/AI/NIAID NIH HHS/United States
- AI57831/AI/NIAID NIH HHS/United States
- AI036629/AI/NIAID NIH HHS/United States
- R01 AI057831/AI/NIAID NIH HHS/United States
- P41 RR000954/RR/NCRR NIH HHS/United States
- R43 AI084243/AI/NIAID NIH HHS/United States
- R01 AI041930/AI/NIAID NIH HHS/United States
- R01 GM079498/GM/NIGMS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- R21 AI073142/AI/NIAID NIH HHS/United States
- AI073142/AI/NIAID NIH HHS/United States
- R01 AI036629-18/AI/NIAID NIH HHS/United States
- R21 AI075931/AI/NIAID NIH HHS/United States
- GM079498/GM/NIGMS NIH HHS/United States
- P41RR000954/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources

