Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism
- PMID: 21145505
- PMCID: PMC3010389
- DOI: 10.1016/j.devcel.2010.10.022
Bmp signaling regulates myocardial differentiation from cardiac progenitors through a MicroRNA-mediated mechanism
Abstract
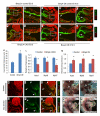
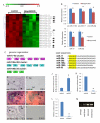
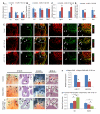
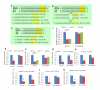
MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression posttranscriptionally. We investigated the hypothesis that bone morphogenetic protein (Bmp) signaling regulates miRNAs in cardiac progenitor cells. Bmp2 and Bmp4 regulate OFT myocardial differentiation via regulation of the miRNA-17-92 cluster. In Bmp mutant embryos, myocardial differentiation was delayed, and multiple miRNAs encoded by miRNA-17-92 were reduced. We uncovered functional miRNA-17-92 seed sequences within the 3' UTR of cardiac progenitor genes such as Isl1 and Tbx1. In both Bmp and miRNA-17-92 mutant embryos, Isl1 and Tbx1 expression failed to be correctly downregulated. Transfection experiments indicated that miRNA-17 and miRNA-20a directly repressed Isl1 and Tbx1. Genetic interaction studies uncovered a synergistic interaction between miRNA-17-92 cluster and Bmp4, providing direct in vivo evidence for the Bmp-miRNA-17-92 regulatory pathway. Our findings indicate that Bmp signaling directly regulates a miRNA-mediated effector mechanism that downregulates cardiac progenitor genes and enhances myocardial differentiation.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Comment in
-
MicroRNAs in a cardiac loop: progenitor or myocyte?Dev Cell. 2010 Dec 14;19(6):787-8. doi: 10.1016/j.devcel.2010.11.015. Dev Cell. 2010. PMID: 21145492 Free PMC article.
Similar articles
-
Bmp Signaling Regulates Hand1 in a Dose-Dependent Manner during Heart Development.Int J Mol Sci. 2021 Sep 11;22(18):9835. doi: 10.3390/ijms22189835. Int J Mol Sci. 2021. PMID: 34576009 Free PMC article.
-
Isl1Cre reveals a common Bmp pathway in heart and limb development.Development. 2006 Apr;133(8):1575-85. doi: 10.1242/dev.02322. Development. 2006. PMID: 16556916 Free PMC article.
-
Bmp signaling exerts opposite effects on cardiac differentiation.Circ Res. 2012 Feb 17;110(4):578-87. doi: 10.1161/CIRCRESAHA.111.261172. Epub 2012 Jan 12. Circ Res. 2012. PMID: 22247485 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
BMP signalling in skeletal development, disease and repair.Nat Rev Endocrinol. 2016 Apr;12(4):203-21. doi: 10.1038/nrendo.2016.12. Epub 2016 Feb 19. Nat Rev Endocrinol. 2016. PMID: 26893264 Review.
Cited by
-
Developmental epigenetics of the murine secondary palate.ILAR J. 2012;53(3-4):240-52. doi: 10.1093/ilar.53.3-4.240. ILAR J. 2012. PMID: 23744964 Free PMC article. Review.
-
Ascl2 knockdown results in tumor growth arrest by miRNA-302b-related inhibition of colon cancer progenitor cells.PLoS One. 2012;7(2):e32170. doi: 10.1371/journal.pone.0032170. Epub 2012 Feb 23. PLoS One. 2012. PMID: 22384170 Free PMC article.
-
Irx4 Marks a Multipotent, Ventricular-Specific Progenitor Cell.Stem Cells. 2016 Dec;34(12):2875-2888. doi: 10.1002/stem.2486. Epub 2016 Sep 13. Stem Cells. 2016. PMID: 27570947 Free PMC article.
-
miRNAs in Heart Development and Disease.Int J Mol Sci. 2024 Jan 30;25(3):1673. doi: 10.3390/ijms25031673. Int J Mol Sci. 2024. PMID: 38338950 Free PMC article. Review.
-
Investigation of the Role of BMP2 and -4 in ASD, VSD and Complex Congenital Heart Disease.Diagnostics (Basel). 2023 Aug 21;13(16):2717. doi: 10.3390/diagnostics13162717. Diagnostics (Basel). 2023. PMID: 37627976 Free PMC article.
References
-
- Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. - PubMed
-
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL064658-11/HL/NHLBI NIH HHS/United States
- T32 DE15355-04/DE/NIDCR NIH HHS/United States
- R01 DE012324-13/DE/NIDCR NIH HHS/United States
- R01 HL064658/HL/NHLBI NIH HHS/United States
- R01 DE012324/DE/NIDCR NIH HHS/United States
- R01 HL093484-02/HL/NHLBI NIH HHS/United States
- CA096824/CA/NCI NIH HHS/United States
- R01 CA096824/CA/NCI NIH HHS/United States
- R01 HL093484/HL/NHLBI NIH HHS/United States
- R01HL093484-01/HL/NHLBI NIH HHS/United States
- 2R01DE12324-12/DE/NIDCR NIH HHS/United States
- T32 DE015355/DE/NIDCR NIH HHS/United States
- R56 CA096824/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

