Superresolution imaging of chemical synapses in the brain
- PMID: 21144999
- PMCID: PMC3057101
- DOI: 10.1016/j.neuron.2010.11.021
Superresolution imaging of chemical synapses in the brain
Abstract
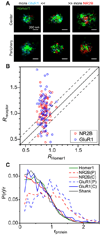
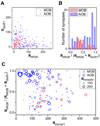
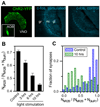
Determination of the molecular architecture of synapses requires nanoscopic image resolution and specific molecular recognition, a task that has so far defied many conventional imaging approaches. Here, we present a superresolution fluorescence imaging method to visualize the molecular architecture of synapses in the brain. Using multicolor, three-dimensional stochastic optical reconstruction microscopy, the distributions of synaptic proteins can be measured with nanometer precision. Furthermore, the wide-field, volumetric imaging method enables high-throughput, quantitative analysis of a large number of synapses from different brain regions. To demonstrate the capabilities of this approach, we have determined the organization of ten protein components of the presynaptic active zone and the postsynaptic density. Variations in synapse morphology, neurotransmitter receptor composition, and receptor distribution were observed both among synapses and across different brain regions. Combination with optogenetics further allowed molecular events associated with synaptic plasticity to be resolved at the single-synapse level.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
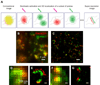
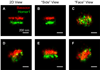
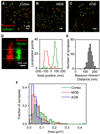
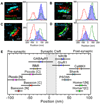
Similar articles
-
Imaging tripartite synapses using super-resolution microscopy.Methods. 2020 Mar 1;174:81-90. doi: 10.1016/j.ymeth.2019.05.024. Epub 2019 May 31. Methods. 2020. PMID: 31153907 Free PMC article.
-
Illuminating Relationships Between the Pre- and Post-synapse.Front Neural Circuits. 2020 Apr 2;14:9. doi: 10.3389/fncir.2020.00009. eCollection 2020. Front Neural Circuits. 2020. PMID: 32308573 Free PMC article. Review.
-
Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy.Science. 2008 Feb 8;319(5864):810-3. doi: 10.1126/science.1153529. Epub 2008 Jan 3. Science. 2008. PMID: 18174397 Free PMC article.
-
Mapping the connectivity of serotonin transporter immunoreactive axons to excitatory and inhibitory neurochemical synapses in the mouse limbic brain.Brain Struct Funct. 2017 Apr;222(3):1297-1314. doi: 10.1007/s00429-016-1278-x. Epub 2016 Aug 2. Brain Struct Funct. 2017. PMID: 27485750 Free PMC article.
-
Imaging of molecular dynamics regulated by electrical activities in neural circuits and in synapses.Neurosignals. 2008;16(4):260-77. doi: 10.1159/000123037. Epub 2008 Jul 18. Neurosignals. 2008. PMID: 18635943 Review.
Cited by
-
Engineered fast-dissociating antibody fragments for multiplexed super-resolution microscopy.Cell Rep Methods. 2022 Sep 20;2(10):100301. doi: 10.1016/j.crmeth.2022.100301. eCollection 2022 Oct 24. Cell Rep Methods. 2022. PMID: 36313806 Free PMC article.
-
Permeabilization-free en bloc immunohistochemistry for correlative microscopy.Elife. 2021 May 13;10:e63392. doi: 10.7554/eLife.63392. Elife. 2021. PMID: 33983117 Free PMC article.
-
Automated analysis of a diverse synapse population.PLoS Comput Biol. 2013;9(3):e1002976. doi: 10.1371/journal.pcbi.1002976. Epub 2013 Mar 28. PLoS Comput Biol. 2013. PMID: 23555213 Free PMC article.
-
Pten Deletion Promotes Regrowth of Corticospinal Tract Axons 1 Year after Spinal Cord Injury.J Neurosci. 2015 Jul 1;35(26):9754-63. doi: 10.1523/JNEUROSCI.3637-14.2015. J Neurosci. 2015. PMID: 26134657 Free PMC article.
-
Imaging nanometre-scale structure in cells using in situ aberration correction.J Microsc. 2012 Oct;248(1):90-101. doi: 10.1111/j.1365-2818.2012.03654.x. Epub 2012 Aug 20. J Microsc. 2012. PMID: 22906048 Free PMC article.
References
-
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. - PubMed
-
- Arthur CP, Serrell DB, Pagratis M, Potter DL, Finch DS, Stowell MH. Electron tomographic methods for studying the chemical synapse. Methods Cell Biol. 2007;79:241–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

