Astrocyte elevated gene-1 induces protective autophagy
- PMID: 21127263
- PMCID: PMC3009793
- DOI: 10.1073/pnas.1009479107
Astrocyte elevated gene-1 induces protective autophagy
Abstract
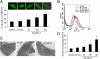
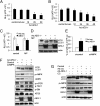
Astrocyte-elevated gene-1 (AEG-1) expression increases in multiple cancers and plays a crucial role in oncogenic transformation and angiogenesis, which are essential components in tumor cell development, growth, and progression to metastasis. Moreover, AEG-1 directly contributes to resistance to chemotherapeutic drugs, another important hallmark of aggressive cancers. In the present study, we document that AEG-1 mediates protective autophagy, an important regulator of cancer survival under metabolic stress and resistance to apoptosis, which may underlie its significant cancer-promoting properties. AEG-1 induces noncanonical autophagy involving an increase in expression of ATG5. AEG-1 decreases the ATP/AMP ratio, resulting in diminished cellular metabolism and activation of AMP kinase, which induces AMPK/mammalian target of rapamycin-dependent autophagy. Inhibition of AMPK by siAMPK or compound C decreases expression of ATG5, ultimately attenuating AEG-1-induced autophagy. AEG-1 protects normal cells from serum starvation-induced death through protective autophagy, and inhibition of AEG-1-induced autophagy results in serum starvation-induced cell death. We also show that AEG-1-mediated chemoresistance is because of protective autophagy and inhibition of AEG-1 results in a decrease in protective autophagy and chemosensitization of cancer cells. In summary, the present study reveals a previously unknown aspect of AEG-1 function by identifying it as a potential regulator of protective autophagy, an important feature of AEG-1 that may contribute to its tumor-promoting properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

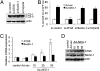


Similar articles
-
Diindolylmethane and its halogenated derivatives induce protective autophagy in human prostate cancer cells via induction of the oncogenic protein AEG-1 and activation of AMP-activated protein kinase (AMPK).Cell Signal. 2017 Dec;40:172-182. doi: 10.1016/j.cellsig.2017.09.006. Epub 2017 Sep 18. Cell Signal. 2017. PMID: 28923415
-
AEG-1 is involved in hypoxia-induced autophagy and decreases chemosensitivity in T-cell lymphoma.Mol Med. 2018 Jul 9;24(1):35. doi: 10.1186/s10020-018-0033-6. Mol Med. 2018. PMID: 30134829 Free PMC article.
-
Drug resistance mediated by AEG-1/MTDH/LYRIC.Adv Cancer Res. 2013;120:135-57. doi: 10.1016/B978-0-12-401676-7.00005-X. Adv Cancer Res. 2013. PMID: 23889990 Free PMC article. Review.
-
Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis.Proc Natl Acad Sci U S A. 2009 Dec 15;106(50):21300-5. doi: 10.1073/pnas.0910936106. Epub 2009 Nov 25. Proc Natl Acad Sci U S A. 2009. PMID: 19940250 Free PMC article.
-
Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration.Pharmacol Ther. 2007 May;114(2):155-70. doi: 10.1016/j.pharmthera.2007.01.010. Epub 2007 Feb 24. Pharmacol Ther. 2007. PMID: 17397930 Free PMC article. Review.
Cited by
-
Lipid droplet density alters the early innate immune response to viral infection.PLoS One. 2018 Jan 2;13(1):e0190597. doi: 10.1371/journal.pone.0190597. eCollection 2018. PLoS One. 2018. PMID: 29293661 Free PMC article.
-
MiR-23a modulates X-linked inhibitor of apoptosis-mediated autophagy in human luminal breast cancer cell lines.Oncotarget. 2017 Sep 19;8(46):80709-80721. doi: 10.18632/oncotarget.21080. eCollection 2017 Oct 6. Oncotarget. 2017. PMID: 29113338 Free PMC article.
-
Spatial expression patterns of autophagy genes in the eye lens and induction of autophagy in lens cells.Mol Vis. 2012;18:1773-86. Epub 2012 Jun 30. Mol Vis. 2012. PMID: 22815631 Free PMC article.
-
Identification of staphylococcal nuclease domain-containing 1 (SND1) as a Metadherin-interacting protein with metastasis-promoting functions.J Biol Chem. 2011 Jun 3;286(22):19982-92. doi: 10.1074/jbc.M111.240077. Epub 2011 Apr 8. J Biol Chem. 2011. PMID: 21478147 Free PMC article.
-
Serum-reduced media impacts on cell viability and protein expression in human lung epithelial cells.J Cell Physiol. 2019 Jun;234(6):7718-7724. doi: 10.1002/jcp.27890. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515823 Free PMC article.
References
-
- Levine B, Klionsky DJ. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. - PubMed
-
- Lum JJ, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. - PubMed
-
- Qu X, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. - PubMed
-
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

