Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice
- PMID: 21126175
- PMCID: PMC3113450
- DOI: 10.1089/ars.2010.3587
Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice
Abstract
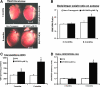
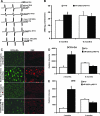
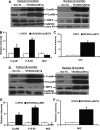
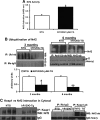
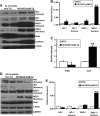
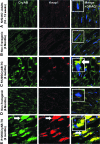
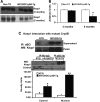
Inheritable missense mutations in small molecular weight heat-shock proteins (HSP) with chaperone-like properties promote self-oligomerization, protein aggregation, and pathologic states such as hypertrophic cardiomyopathy in humans. We recently described that human mutant αB-crystallin (hR120GCryAB) overexpression that caused protein aggregation cardiomyopathy (PAC) was genetically linked to dysregulation of the antioxidant system and reductive stress (RS) in mice. However, the molecular mechanism that induces RS remains only partially understood. Here we define a critical role for the regulatory nuclear erythroid 2-related factor 2 (Nrf2)-Kelch-like ECH-associated protein (Keap1) pathway--the master transcriptional controller of antioxidants, in the pathogenesis of PAC and RS. In myopathic mice, increased reactive oxygen species signaling during compensatory hypertrophy (i.e., 3 months) was associated with upregulation of key antioxidants in a manner consistent with Nrf2/antioxidant response element (ARE)-dependent transactivation. In transcription factor assays, we further demonstrate increased binding of Nrf2 to ARE during the development of cardiomyopathy. Of interest, we show that the negative regulator Keap1 was predominantly sequestrated in protein aggregates (at 6 months), suggesting that sustained nuclear translocation of activated Nrf2 may be a contributing mechanism for RS. Our findings implicate a novel pathway for therapeutic targeting and abrogating RS linked to experimental cardiomyopathy in humans. Antioxid.
Figures
Similar articles
-
Restoration of Nrf2 Signaling Normalizes the Regenerative Niche.Diabetes. 2016 Mar;65(3):633-46. doi: 10.2337/db15-0453. Epub 2015 Dec 8. Diabetes. 2016. PMID: 26647385 Free PMC article.
-
Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium.Free Radic Biol Med. 2012 Jan 15;52(2):366-76. doi: 10.1016/j.freeradbiomed.2011.10.440. Epub 2011 Oct 20. Free Radic Biol Med. 2012. PMID: 22051043 Free PMC article.
-
Increased Nrf2 activation in livers from Keap1-knockdown mice increases expression of cytoprotective genes that detoxify electrophiles more than those that detoxify reactive oxygen species.Toxicol Sci. 2009 Mar;108(1):35-47. doi: 10.1093/toxsci/kfn267. Epub 2009 Jan 6. Toxicol Sci. 2009. PMID: 19129213 Free PMC article.
-
The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress.J Biol Chem. 2009 May 15;284(20):13291-5. doi: 10.1074/jbc.R900010200. Epub 2009 Jan 30. J Biol Chem. 2009. PMID: 19182219 Free PMC article. Review.
-
Responses to reductive stress in the cardiovascular system.Free Radic Biol Med. 2017 Aug;109:114-124. doi: 10.1016/j.freeradbiomed.2016.12.006. Epub 2016 Dec 8. Free Radic Biol Med. 2017. PMID: 27940350 Free PMC article. Review.
Cited by
-
Small heat shock proteins in redox metabolism: implications for cardiovascular diseases.Int J Biochem Cell Biol. 2012 Oct;44(10):1632-45. doi: 10.1016/j.biocel.2012.06.006. Epub 2012 Jun 15. Int J Biochem Cell Biol. 2012. PMID: 22710345 Free PMC article. Review.
-
Cannabinoid-Induced Autophagy and Heme Oxygenase-1 Determine the Fate of Adipose Tissue-Derived Mesenchymal Stem Cells under Stressful Conditions.Cells. 2020 Oct 15;9(10):2298. doi: 10.3390/cells9102298. Cells. 2020. PMID: 33076330 Free PMC article.
-
The Interplay between Autophagy and Redox Signaling in Cardiovascular Diseases.Cells. 2022 Apr 2;11(7):1203. doi: 10.3390/cells11071203. Cells. 2022. PMID: 35406767 Free PMC article. Review.
-
Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics.Free Radic Biol Med. 2014 Jun;71:196-207. doi: 10.1016/j.freeradbiomed.2014.03.025. Epub 2014 Mar 26. Free Radic Biol Med. 2014. PMID: 24681256 Free PMC article. Review.
-
The KEAP1-NRF2 System: a Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis.Physiol Rev. 2018 Jul 1;98(3):1169-1203. doi: 10.1152/physrev.00023.2017. Physiol Rev. 2018. PMID: 29717933 Free PMC article. Review.
References
-
- Cardounel AJ. Xia Y. Zweier JL. Endogenous methylarginines modulate superoxide as well as nitric oxide generation from neuronal nitric-oxide synthase: differences in the effects of monomethyl- and dimethylarginines in the presence and absence of tetrahydrobiopterin. J Biol Chem. 2005;280:7540–7549. - PubMed
-
- Chen Q. Liu JB. Horak KM. Zheng H. Kumarapeli AR. Li J. Li F. Gerdes AM. Wawrousek EF. Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–1026. - PubMed
-
- Delbosc S. Paizanis E. Magous R. Araiz C. Dimo T. Cristol JP. Cros G. Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

