Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41
- PMID: 21124990
- PMCID: PMC2987821
- DOI: 10.1371/journal.ppat.1001195
Crystal structure and size-dependent neutralization properties of HK20, a human monoclonal antibody binding to the highly conserved heptad repeat 1 of gp41
Abstract
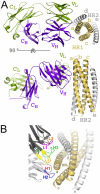
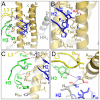
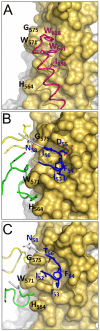
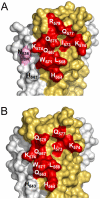
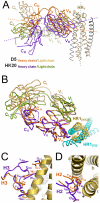
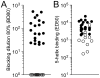
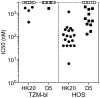
The human monoclonal antibody (mAb) HK20 neutralizes a broad spectrum of primary HIV-1 isolates by targeting the highly conserved heptad repeat 1 (HR1) of gp41, which is transiently exposed during HIV-1 entry. Here we present the crystal structure of the HK20 Fab in complex with a gp41 mimetic 5-Helix at 2.3 Å resolution. HK20 employs its heavy chain CDR H2 and H3 loops to bind into a conserved hydrophobic HR1 pocket that is occupied by HR2 residues in the gp41 post fusion conformation. Compared to the previously described HR1-specific mAb D5, HK20 approaches its epitope with a different angle which might favor epitope access and thus contribute to its higher neutralization breadth and potency. Comparison of the neutralization activities of HK20 IgG, Fab and scFv employing both single cycle and multiple cycle neutralization assays revealed much higher potencies for the smaller Fab and scFv over IgG, implying that the target site is difficult to access for complete antibodies. Nevertheless, two thirds of sera from HIV-1 infected individuals contain significant titers of HK20-inhibiting antibodies. The breadth of neutralization of primary isolates across all clades, the higher potencies for C-clade viruses and the targeting of a distinct site as compared to the fusion inhibitor T-20 demonstrate the potential of HK20 scFv as a therapeutic tool.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Structural basis of HIV-1 neutralization by affinity matured Fabs directed against the internal trimeric coiled-coil of gp41.PLoS Pathog. 2010 Nov 11;6(11):e1001182. doi: 10.1371/journal.ppat.1001182. PLoS Pathog. 2010. PMID: 21085615 Free PMC article.
-
Affinity maturation and characterization of a human monoclonal antibody against HIV-1 gp41.MAbs. 2009 Sep-Oct;1(5):462-74. doi: 10.4161/mabs.1.5.9214. Epub 2009 Sep 8. MAbs. 2009. PMID: 20065653 Free PMC article.
-
Binding of HIV-1 gp41-directed neutralizing and non-neutralizing fragment antibody binding domain (Fab) and single chain variable fragment (ScFv) antibodies to the ectodomain of gp41 in the pre-hairpin and six-helix bundle conformations.PLoS One. 2014 Aug 8;9(8):e104683. doi: 10.1371/journal.pone.0104683. eCollection 2014. PLoS One. 2014. PMID: 25105806 Free PMC article.
-
Short communication: In vitro synergy between peptides or neutralizing antibodies targeting the N- and C-terminal heptad repeats of HIV Type 1 gp41.AIDS Res Hum Retroviruses. 2008 Dec;24(12):1537-44. doi: 10.1089/aid.2008.0129. AIDS Res Hum Retroviruses. 2008. PMID: 19102685 Review.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
Cited by
-
HIV-1 envelope glycoprotein structure.Curr Opin Struct Biol. 2013 Apr;23(2):268-76. doi: 10.1016/j.sbi.2013.03.007. Epub 2013 Apr 18. Curr Opin Struct Biol. 2013. PMID: 23602427 Free PMC article. Review.
-
VH1-69 antiviral broadly neutralizing antibodies: genetics, structures, and relevance to rational vaccine design.Curr Opin Virol. 2019 Feb;34:149-159. doi: 10.1016/j.coviro.2019.02.004. Epub 2019 Mar 16. Curr Opin Virol. 2019. PMID: 30884330 Free PMC article. Review.
-
Molecular dynamics studies of the inhibitor C34 binding to the wild-type and mutant HIV-1 gp41: inhibitory and drug resistant mechanism.PLoS One. 2014 Nov 13;9(11):e111923. doi: 10.1371/journal.pone.0111923. eCollection 2014. PLoS One. 2014. PMID: 25393106 Free PMC article.
-
Restricted, canonical, stereotyped and convergent immunoglobulin responses.Philos Trans R Soc Lond B Biol Sci. 2015 Sep 5;370(1676):20140238. doi: 10.1098/rstb.2014.0238. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26194752 Free PMC article. Review.
-
Rational design of vaccines to elicit broadly neutralizing antibodies to HIV-1.Cold Spring Harb Perspect Med. 2011 Sep;1(1):a007278. doi: 10.1101/cshperspect.a007278. Cold Spring Harb Perspect Med. 2011. PMID: 22229123 Free PMC article. Review.
References
-
- Karlsson Hedestam GB, Fouchier RAM, Phogat S, Burton DR, Sodroski J, et al. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. 2008;6:143–155. - PubMed
-
- Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, et al. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

