Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication
- PMID: 21123650
- PMCID: PMC2994037
- DOI: 10.1101/gad.1978310
Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication
Abstract
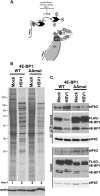
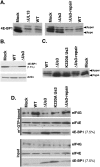
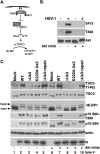
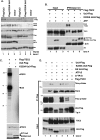
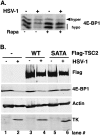
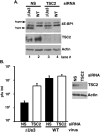
All viruses require cellular ribosomes to translate their mRNAs. Viruses producing methyl-7 (m⁷) GTP-capped mRNAs, like Herpes Simplex Virus-1 (HSV-1), stimulate cap-dependent translation by activating mTORC1 to inhibit the translational repressor 4E-binding protein 1 (4E-BP1). Here, we establish that the HSV-1 kinase Us3 masquerades as Akt to activate mTORC1. Remarkably, Us3 displays no sequence homology with the cellular kinase Akt, yet directly phosphorylates tuberous sclerosis complex 2 (TSC2) on the same sites as Akt. TSC2 depletion rescued Us3-deficient virus replication, establishing that Us3 enhances replication by phosphorylating TSC2 to constitutively activate mTORC1, effectively bypassing S6K-mediated feedback inhibition. Moreover, Us3 stimulated Akt substrate phosphorylation in infected cells, including FOXO1 and GSK3. Thus, HSV-1 encodes an Akt surrogate with overlapping substrate specificity to activate mTORC1, stimulating translation and virus replication. This establishes Us3 as a unique viral kinase with promising drug development potential.
Figures

Comment in
-
Herpes Simplex Virus is Akt-ing in translational control.Genes Dev. 2010 Dec 1;24(23):2583-6. doi: 10.1101/gad.2004510. Genes Dev. 2010. PMID: 21123644 Free PMC article.
Similar articles
-
Herpes Simplex Virus is Akt-ing in translational control.Genes Dev. 2010 Dec 1;24(23):2583-6. doi: 10.1101/gad.2004510. Genes Dev. 2010. PMID: 21123644 Free PMC article.
-
Remodeling mTORC1 Responsiveness to Amino Acids by the Herpes Simplex Virus UL46 and Us3 Gene Products Supports Replication during Nutrient Insufficiency.J Virol. 2018 Nov 27;92(24):e01377-18. doi: 10.1128/JVI.01377-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282708 Free PMC article.
-
A herpesvirus kinase that masquerades as Akt: you don't have to look like Akt, to act like it.Cell Cycle. 2011 Jul 1;10(13):2064-8. doi: 10.4161/cc.10.13.16242. Epub 2011 Jul 1. Cell Cycle. 2011. PMID: 21606676 Free PMC article.
-
Us3 Protein Kinase Encoded by HSV: The Precise Function and Mechanism on Viral Life Cycle.Adv Exp Med Biol. 2018;1045:45-62. doi: 10.1007/978-981-10-7230-7_3. Adv Exp Med Biol. 2018. PMID: 29896662 Review.
-
A complex interplay between Akt, TSC2 and the two mTOR complexes.Biochem Soc Trans. 2009 Feb;37(Pt 1):217-22. doi: 10.1042/BST0370217. Biochem Soc Trans. 2009. PMID: 19143635 Free PMC article. Review.
Cited by
-
Adapting the Stress Response: Viral Subversion of the mTOR Signaling Pathway.Viruses. 2016 May 24;8(6):152. doi: 10.3390/v8060152. Viruses. 2016. PMID: 27231932 Free PMC article. Review.
-
Viral MicroRNAs in Herpes Simplex Virus 1 Pathobiology.Curr Pharm Des. 2024;30(9):649-665. doi: 10.2174/0113816128286469240129100313. Curr Pharm Des. 2024. PMID: 38347772 Review.
-
Current and Emerging Therapies for Ocular Herpes Simplex Virus Type-1 Infections.Microorganisms. 2019 Oct 10;7(10):429. doi: 10.3390/microorganisms7100429. Microorganisms. 2019. PMID: 31658632 Free PMC article. Review.
-
Active-site mTOR inhibitors augment HSV1-dICP0 infection in cancer cells via dysregulated eIF4E/4E-BP axis.PLoS Pathog. 2018 Aug 23;14(8):e1007264. doi: 10.1371/journal.ppat.1007264. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30138450 Free PMC article.
-
The US3 Kinase of Herpes Simplex Virus Phosphorylates the RNA Sensor RIG-I To Suppress Innate Immunity.J Virol. 2022 Feb 23;96(4):e0151021. doi: 10.1128/JVI.01510-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34935440 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
