The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways
- PMID: 21123379
- PMCID: PMC3028889
- DOI: 10.1128/JVI.01608-10
The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways
Abstract
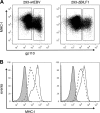
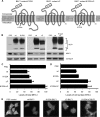
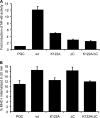
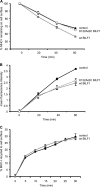
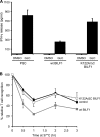
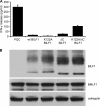
Despite triggering strong immune responses, Epstein-Barr virus (EBV) has colonized more than 90% of the adult human population. Successful persistence of EBV depends on the establishment of a balance between host immune responses and viral immune evasion. Here we have extended our studies on the EBV-encoded BILF1 protein, which was recently identified as an immunoevasin that functions by enhancing degradation of major histocompatibility complex class I (MHC-I) antigens via lysosomes. We now demonstrate that disruption of the EKT signaling motif of BILF1 by a K122A mutation impairs the ability of BILF1 to enhance endocytosis of surface MHC-I molecules, while subsequent lysosomal degradation was impaired by deletion of the 21-residue C-terminal tail of BILF1. Furthermore, we identified another mechanism of BILF1 immunomodulation: it targets newly synthesized MHC-I/peptide complexes en route to the cell surface. Importantly, although the diversion of MHC-I on the exocytic pathway caused a relatively modest reduction in cell surface MHC-I, presentation of endogenously processed target peptides to immune CD8(+) effector T cells was reduced by around 65%. The immune-modulating functions of BILF1 in the context of the whole virus were confirmed in cells lytically infected with a recombinant EBV in which BILF1 was deleted. This study therefore extends our initial observations on BILF1 to show that this immunoevasin can target MHC-I antigen presentation via both the exocytic and endocytic trafficking pathways. The results also emphasize the merits of including functional T cell recognition assays to gain a more complete picture of immunoevasin effects on the antigen presentation pathway.
Figures

Similar articles
-
The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation.PLoS Pathog. 2009 Jan;5(1):e1000255. doi: 10.1371/journal.ppat.1000255. Epub 2009 Jan 2. PLoS Pathog. 2009. PMID: 19119421 Free PMC article.
-
Distinct Roles of Extracellular Domains in the Epstein-Barr Virus-Encoded BILF1 Receptor for Signaling and Major Histocompatibility Complex Class I Downregulation.mBio. 2019 Jan 15;10(1):e01707-18. doi: 10.1128/mBio.01707-18. mBio. 2019. PMID: 30647152 Free PMC article.
-
The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II.J Virol. 2015 Oct 14;90(1):356-67. doi: 10.1128/JVI.02183-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468525 Free PMC article.
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Endocytic Recycling of MHC Class I Molecules in Non-professional Antigen Presenting and Dendritic Cells.Front Immunol. 2019 Jan 7;9:3098. doi: 10.3389/fimmu.2018.03098. eCollection 2018. Front Immunol. 2019. PMID: 30666258 Free PMC article. Review.
Cited by
-
Evasion of adaptive and innate immune response mechanisms by γ-herpesviruses.Curr Opin Virol. 2013 Jun;3(3):285-95. doi: 10.1016/j.coviro.2013.05.011. Epub 2013 Jun 2. Curr Opin Virol. 2013. PMID: 23735334 Free PMC article. Review.
-
Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses.Annu Rev Virol. 2022 Sep 29;9(1):329-351. doi: 10.1146/annurev-virology-100220-113942. Epub 2022 Jun 7. Annu Rev Virol. 2022. PMID: 35671566 Free PMC article. Review.
-
Viral and cellular oncogenes promote immune evasion.Oncogene. 2022 Feb;41(7):921-929. doi: 10.1038/s41388-021-02145-1. Epub 2022 Jan 13. Oncogene. 2022. PMID: 35022539 Free PMC article. Review.
-
High Levels of Class I Major Histocompatibility Complex mRNA Are Present in Epstein-Barr Virus-Associated Gastric Adenocarcinomas.Cells. 2020 Feb 21;9(2):499. doi: 10.3390/cells9020499. Cells. 2020. PMID: 32098275 Free PMC article.
-
The Role of NK Cells in EBV Infection and Related Diseases: Current Understanding and Hints for Novel Therapies.Cancers (Basel). 2023 Mar 22;15(6):1914. doi: 10.3390/cancers15061914. Cancers (Basel). 2023. PMID: 36980798 Free PMC article. Review.
References
-
- Atkinson, P. G., H. J. Coope, M. Rowe, and S. C. Ley. 2003. Latent membrane protein 1 of Epstein-Barr virus stimulates processing of NF-kappa B2 p100 to p52. J. Biol. Chem. 278:51134-51142. - PubMed
-
- Barnstable, C. J., et al. 1978. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell 14:9-20. - PubMed
-
- Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

