Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis
- PMID: 21119045
- PMCID: PMC3032481
- DOI: 10.1104/pp.110.164640
Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis
Abstract
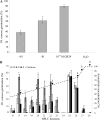
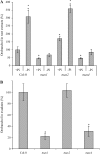
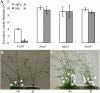
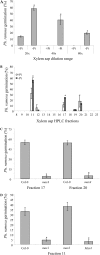
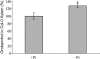
The biosynthesis of the recently identified novel class of plant hormones, strigolactones, is up-regulated upon phosphate deficiency in many plant species. It is generally accepted that the evolutionary origin of strigolactone up-regulation is their function as a rhizosphere signal that stimulates hyphal branching of arbuscular mycorrhizal fungi. In this work, we demonstrate that this induction is conserved in Arabidopsis (Arabidopsis thaliana), although Arabidopsis is not a host for arbuscular mycorrhizal fungi. We demonstrate that the increase in strigolactone production contributes to the changes in shoot architecture observed in response to phosphate deficiency. Using high-performance liquid chromatography, column chromatography, and multiple reaction monitoring-liquid chromatography-tandem mass spectrometry analysis, we identified two strigolactones (orobanchol and orobanchyl acetate) in Arabidopsis and have evidence of the presence of a third (5-deoxystrigol). We show that at least one of them (orobanchol) is strongly reduced in the putative strigolactone biosynthetic mutants more axillary growth1 (max1) and max4 but not in the signal transduction mutant max2. Orobanchol was also detected in xylem sap and up-regulated under phosphate deficiency, which is consistent with the idea that root-derived strigolactones are transported to the shoot, where they regulate branching. Moreover, two additional putative strigolactone-like compounds were detected in xylem sap, one of which was not detected in root exudates. Together, these results show that xylem-transported strigolactones contribute to the regulation of shoot architectural response to phosphate-limiting conditions.
Figures
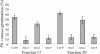


Similar articles
-
Strigolactones: Internal and external signals in plant symbioses?Plant Signal Behav. 2013 Mar;8(3):e23168. doi: 10.4161/psb.23168. Epub 2013 Jan 8. Plant Signal Behav. 2013. PMID: 23299321 Free PMC article.
-
Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane.PLoS Biol. 2013;11(1):e1001474. doi: 10.1371/journal.pbio.1001474. Epub 2013 Jan 29. PLoS Biol. 2013. PMID: 23382651 Free PMC article.
-
LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis.Proc Natl Acad Sci U S A. 2016 May 31;113(22):6301-6. doi: 10.1073/pnas.1601729113. Epub 2016 May 18. Proc Natl Acad Sci U S A. 2016. PMID: 27194725 Free PMC article.
-
Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds.Sci China C Life Sci. 2009 Aug;52(8):693-700. doi: 10.1007/s11427-009-0104-6. Epub 2009 Aug 29. Sci China C Life Sci. 2009. PMID: 19727586 Review.
-
Strigolactones: structures and biological activities.Pest Manag Sci. 2009 May;65(5):467-70. doi: 10.1002/ps.1726. Pest Manag Sci. 2009. PMID: 19222028 Review.
Cited by
-
Strigolactones: Internal and external signals in plant symbioses?Plant Signal Behav. 2013 Mar;8(3):e23168. doi: 10.4161/psb.23168. Epub 2013 Jan 8. Plant Signal Behav. 2013. PMID: 23299321 Free PMC article.
-
The importance of strigolactone transport regulation for symbiotic signaling and shoot branching.Planta. 2016 Jun;243(6):1351-60. doi: 10.1007/s00425-016-2503-9. Epub 2016 Apr 4. Planta. 2016. PMID: 27040840 Free PMC article. Review.
-
Strigolactone signaling is required for auxin-dependent stimulation of secondary growth in plants.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):20242-7. doi: 10.1073/pnas.1111902108. Epub 2011 Nov 28. Proc Natl Acad Sci U S A. 2011. PMID: 22123958 Free PMC article.
-
Role of Strigolactones: Signalling and Crosstalk with Other Phytohormones.Open Life Sci. 2020 Apr 10;15:217-228. doi: 10.1515/biol-2020-0022. eCollection 2020. Open Life Sci. 2020. PMID: 33987478 Free PMC article.
-
Phytohormone signaling and crosstalk in regulating drought stress response in plants.Plant Cell Rep. 2021 Aug;40(8):1305-1329. doi: 10.1007/s00299-021-02683-8. Epub 2021 Mar 22. Plant Cell Rep. 2021. PMID: 33751168 Review.
References
-
- Akiyama K, Matsuzaki K, Hayashi H. (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 - PubMed
-
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P. (2003) Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signaling. Plant Cell Environ 26: 1053–1066
-
- Bates TR, Lynch JP. (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19: 529–538
-
- Bennett T. (2006) The regulation of shoot branching in Arabidopsis thaliana. PhD thesis. University of York, York, UK
-
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

