Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production
- PMID: 21115691
- PMCID: PMC3005235
- DOI: 10.1084/jem.20101326
Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production
Abstract
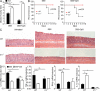
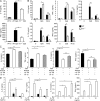
The interleukin 1 receptor (IL-1R) and the Toll-like receptors (TLRs) are highly homologous innate immune receptors that provide the first line of defense against infection. We show that IL-1R type I (IL-1RI) is essential for TLR9-dependent activation of tumor necrosis factor receptor-associated factor 3 (TRAF3) and for production of the antiinflammatory cytokines IL-10 and type I interferon (IFN). Noncanonical K63-linked ubiquitination of TRAF3, which is essential for type I IFN and IL-10 production, was impaired in Il1r1(-/-) CD11c(+) dendritic cells. In contrast, degradative ubiquitination of TRAF3 was not affected in the absence of IL-1R1 signaling. Deubiquitinating enzyme A (DUBA), which selectively cleaves K63-linked ubiquitin chains from TRAF3, was up-regulated in the absence of IL-1R1 signaling. DUBA short interference RNA augmented the TLR9-dependent type I IFN response. Mice deficient in IL-1RI signaling showed reduced expression of IL-10 and type I IFN and increased susceptibility to dextran sulphate sodium-induced colitis and failed to mount a protective type I IFN response after TLR9 ligand (CpG) administration. Our data identifies a new molecular pathway by which IL-1 signaling attenuates TLR9-mediated proinflammatory responses.
Figures

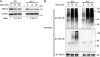
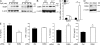
Similar articles
-
DUBA: a deubiquitinase that regulates type I interferon production.Science. 2007 Dec 7;318(5856):1628-32. doi: 10.1126/science.1145918. Epub 2007 Nov 8. Science. 2007. PMID: 17991829
-
Activation of nucleotide-binding oligomerization domain 2 by muramyl dipeptide negatively regulates Toll-like receptor 9-mediated colonic inflammation through the induction of deubiquitinating enzyme A expression.Int Immunol. 2023 Feb 11;35(2):79-94. doi: 10.1093/intimm/dxac045. Int Immunol. 2023. PMID: 36171063
-
Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines.Nat Immunol. 2010 Jan;11(1):70-5. doi: 10.1038/ni.1819. Epub 2009 Nov 8. Nat Immunol. 2010. PMID: 19898473 Free PMC article.
-
Involvement of DNA-PKcs in the type I IFN response to CpG-ODNs in conventional dendritic cells in TLR9-dependent or -independent manners.PLoS One. 2015 Mar 26;10(3):e0121371. doi: 10.1371/journal.pone.0121371. eCollection 2015. PLoS One. 2015. PMID: 25812014 Free PMC article.
-
The autoimmunity-associated gene PTPN22 potentiates toll-like receptor-driven, type 1 interferon-dependent immunity.Immunity. 2013 Jul 25;39(1):111-22. doi: 10.1016/j.immuni.2013.06.013. Epub 2013 Jul 18. Immunity. 2013. PMID: 23871208 Free PMC article.
Cited by
-
DUBA-UBR5 axis: other than transactivation.Cell Res. 2015 Mar;25(3):273-4. doi: 10.1038/cr.2015.13. Epub 2015 Jan 30. Cell Res. 2015. PMID: 25633593 Free PMC article.
-
Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions.Immunol Rev. 2011 Nov;244(1):55-74. doi: 10.1111/j.1600-065X.2011.01055.x. Immunol Rev. 2011. PMID: 22017431 Free PMC article. Review.
-
TRAF3: a novel tumor suppressor gene in macrophages.Macrophage (Houst). 2015 Sep 30;2:e1009. doi: 10.14800/macrophage.1009. Macrophage (Houst). 2015. PMID: 26661944 Free PMC article.
-
Novel anti-inflammatory effects of the IL-1 receptor in kidney myeloid cells following ischemic AKI.Front Mol Biosci. 2024 Apr 17;11:1366259. doi: 10.3389/fmolb.2024.1366259. eCollection 2024. Front Mol Biosci. 2024. PMID: 38693918 Free PMC article.
-
A novel TLR2-triggered signalling crosstalk synergistically intensifies TNF-mediated IL-6 induction.J Cell Mol Med. 2014 Jul;18(7):1344-57. doi: 10.1111/jcmm.12294. Epub 2014 Apr 24. J Cell Mol Med. 2014. PMID: 24758719 Free PMC article.
References
-
- Abe K., Nguyen K.P., Fine S.D., Mo J.H., Shen C., Shenouda S., Corr M., Jung S., Lee J., Eckmann L., Raz E. 2007. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc. Natl. Acad. Sci. USA. 104:17022–17027 10.1073/pnas.0708469104 - DOI - PMC - PubMed
-
- Bresnihan B., Alvaro-Gracia J.M., Cobby M., Doherty M., Domljan Z., Emery P., Nuki G., Pavelka K., Rau R., Rozman B., et al. 1998. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis Rheum. 41:2196–2204 10.1002/1529-0131(199812)41:12<2196::AID-ART15>3.0.CO;2-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

