MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling
- PMID: 21115689
- PMCID: PMC3005225
- DOI: 10.1084/jem.20092641
MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling
Abstract
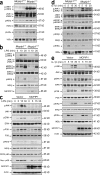
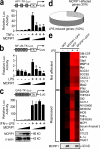
The intensity and duration of macrophage-mediated inflammatory responses are controlled by proteins that modulate inflammatory signaling pathways. MCPIP1 (monocyte chemotactic protein-induced protein 1), a recently identified CCCH Zn finger-containing protein, plays an essential role in controlling macrophage-mediated inflammatory responses. However, its mechanism of action is poorly understood. In this study, we show that MCPIP1 negatively regulates c-Jun N-terminal kinase (JNK) and NF-κB activity by removing ubiquitin moieties from proteins, including TRAF2, TRAF3, and TRAF6. MCPIP1-deficient mice spontaneously developed fatal inflammatory syndrome. Macrophages and splenocytes from MCPIP1(-/-) mice showed elevated expression of inflammatory gene expression, increased JNK and IκB kinase activation, and increased polyubiquitination of TNF receptor-associated factors. In vitro assays directly demonstrated the deubiquitinating activity of purified MCPIP1. Sequence analysis together with serial mutagenesis defined a deubiquitinating enzyme domain and a ubiquitin association domain in MCPIP1. Our results indicate that MCPIP1 is a critical modulator of inflammatory signaling.
Figures





Similar articles
-
Lysine 63-linked polyubiquitination of TAK1 at lysine 158 is required for tumor necrosis factor alpha- and interleukin-1beta-induced IKK/NF-kappaB and JNK/AP-1 activation.J Biol Chem. 2010 Feb 19;285(8):5347-60. doi: 10.1074/jbc.M109.076976. Epub 2009 Dec 28. J Biol Chem. 2010. PMID: 20038579 Free PMC article.
-
TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase.J Biol Chem. 2015 May 22;290(21):13372-85. doi: 10.1074/jbc.M115.643767. Epub 2015 Apr 10. J Biol Chem. 2015. PMID: 25861989 Free PMC article.
-
Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction.J Biol Chem. 2015 Apr 10;290(15):9660-73. doi: 10.1074/jbc.M114.609685. Epub 2015 Feb 25. J Biol Chem. 2015. PMID: 25716317 Free PMC article.
-
Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions.Biochim Biophys Acta. 2012 Oct;1823(10):1905-13. doi: 10.1016/j.bbamcr.2012.06.029. Epub 2012 Jul 4. Biochim Biophys Acta. 2012. PMID: 22771441 Review.
-
MCP-1-induced protein-1, an immune regulator.Protein Cell. 2012 Dec;3(12):903-10. doi: 10.1007/s13238-012-2075-9. Epub 2012 Nov 7. Protein Cell. 2012. PMID: 23132255 Free PMC article. Review.
Cited by
-
Inhibition of 19S proteasome-associated deubiquitinases by metal-containing compounds.Oncoscience. 2015 May 31;2(5):457-66. doi: 10.18632/oncoscience.167. eCollection 2015. Oncoscience. 2015. PMID: 26097878 Free PMC article. Review.
-
How are MCPIP1 and cytokines mutually regulated in cancer-related immunity?Protein Cell. 2020 Dec;11(12):881-893. doi: 10.1007/s13238-020-00739-1. Epub 2020 Jun 16. Protein Cell. 2020. PMID: 32548715 Free PMC article. Review.
-
Curcumin enhances cytotoxic effects of bortezomib in human multiple myeloma H929 cells: potential roles of NF-κB/JNK.Int J Mol Sci. 2012;13(4):4831-4838. doi: 10.3390/ijms13044831. Epub 2012 Apr 16. Int J Mol Sci. 2012. PMID: 22606012 Free PMC article.
-
Circular RNAs in organ injury: recent development.J Transl Med. 2022 Nov 18;20(1):533. doi: 10.1186/s12967-022-03725-9. J Transl Med. 2022. PMID: 36401311 Free PMC article. Review.
-
MCPIP1/regnase-I inhibits simian immunodeficiency virus and is not counteracted by Vpx.J Gen Virol. 2016 Jul;97(7):1693-1698. doi: 10.1099/jgv.0.000482. Epub 2016 Apr 13. J Gen Virol. 2016. PMID: 27075251 Free PMC article.
References
-
- Deng L., Wang C., Spencer E., Yang L., Braun A., You J., Slaughter C., Pickart C., Chen Z.J.. 2000. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 103:351–361. 10.1016/S0092-8674(00)00126-4 - DOI - PubMed
-
- Hadari T., Warms J.V., Rose I.A., Hershko A.. 1992. A ubiquitin C-terminal isopeptidase that acts on polyubiquitin chains. Role in protein degradation. J. Biol. Chem. 267:719–727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

