14S,21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds
- PMID: 21112969
- PMCID: PMC3039401
- DOI: 10.1074/jbc.M110.100388
14S,21R-dihydroxydocosahexaenoic acid remedies impaired healing and mesenchymal stem cell functions in diabetic wounds
Abstract
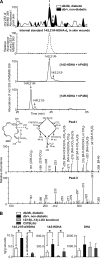
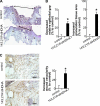
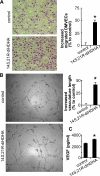
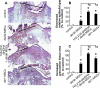
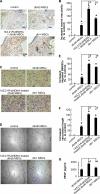
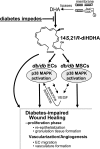
Treatment of diabetes-impaired wound healing remains a major unresolved medical challenge. Here, we identified suppressed formation of a novel reparative lipid mediator 14S,21R-dihydroxydocosa-4Z,7Z,10Z,12E,16Z,19Z-hexaenoic acid (14S,21R-diHDHA) in cutaneous wounds of diabetic db/db mice. These results indicate that diabetes impedes the biosynthetic pathways of 14S,21R-diHDHA in skin wounds. Administration of exogenous 14S,21R-diHDHA to wounds in diabetic animals rescued healing and angiogenesis. When db/db mesenchymal stem cells (MSCs) were administered together with 14S,21R-diHDHA to wounds in diabetic animals, they coacted to accelerate wound re-epithelialization, granulation tissue formation, and synergistically improved vascularization. In the pivotal cellular processes of angiogenesis, 14S,21R-diHDHA enhanced VEGF release, vasculature formation, and migration of db/db dermal microvascular endothelial cells (DMVECs), as well as remedied paracrine angiogenic functions of db/db MSCs, including VEGF secretion and the promotion of DMVEC migration and vasculature formation. Our results show that 14S,21R-diHDHA activates the p38 MAPK pathway in wounds, db/db MSCs, and DMVECs. Overall, the impeded formation of 14S,21R-diHDHA described in this study suggests that diabetes could affect the generation of pro-healing lipid mediators in wound healing. By restoring wound healing and MSC functions, 14S,21R-diHDHA is a new lead for the development of better therapeutics used in treating wounds of diabetics.
Figures
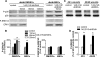
Similar articles
-
Autacoid 14S,21R-dihydroxy-docosahexaenoic acid counteracts diabetic impairment of macrophage prohealing functions.Am J Pathol. 2011 Oct;179(4):1780-91. doi: 10.1016/j.ajpath.2011.06.026. Epub 2011 Aug 10. Am J Pathol. 2011. PMID: 21839062 Free PMC article.
-
Novel 14S,21-dihydroxy-docosahexaenoic acid rescues wound healing and associated angiogenesis impaired by acute ethanol intoxication/exposure.J Cell Biochem. 2010 Oct 1;111(2):266-73. doi: 10.1002/jcb.22709. J Cell Biochem. 2010. PMID: 20506249 Free PMC article.
-
Novel 14,21-dihydroxy-docosahexaenoic acids: structures, formation pathways, and enhancement of wound healing.J Lipid Res. 2010 May;51(5):923-32. doi: 10.1194/jlr.M000059. Epub 2009 Nov 5. J Lipid Res. 2010. PMID: 19965612 Free PMC article.
-
Understanding the multifaceted mechanisms of diabetic wound healing and therapeutic application of stem cells conditioned medium in the healing process.J Tissue Eng Regen Med. 2019 Dec;13(12):2218-2233. doi: 10.1002/term.2966. Epub 2019 Nov 19. J Tissue Eng Regen Med. 2019. PMID: 31648415 Review.
-
A review on different methods to increase the efficiency of mesenchymal stem cell-based wound therapy.Wound Repair Regen. 2019 Nov;27(6):661-671. doi: 10.1111/wrr.12749. Epub 2019 Jul 30. Wound Repair Regen. 2019. PMID: 31298446 Review.
Cited by
-
Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury.Front Immunol. 2013 Jan 30;4:13. doi: 10.3389/fimmu.2013.00013. eCollection 2013. Front Immunol. 2013. PMID: 23386851 Free PMC article.
-
Bactericidal Efficacy of the Combination of Maresin-like Proresolving Mediators and Carbenicillin Action on Biofilm-Forming Burn Trauma Infection-Related Bacteria.Int J Mol Sci. 2024 Feb 28;25(5):2792. doi: 10.3390/ijms25052792. Int J Mol Sci. 2024. PMID: 38474038 Free PMC article.
-
Mesenchymal stem cells and cutaneous wound healing: novel methods to increase cell delivery and therapeutic efficacy.Stem Cell Res Ther. 2016 Mar 9;7:37. doi: 10.1186/s13287-016-0303-6. Stem Cell Res Ther. 2016. PMID: 26960535 Free PMC article. Review.
-
Unsaturated fatty acids induce mesenchymal stem cells to increase secretion of angiogenic mediators.J Cell Physiol. 2012 Sep;227(9):3225-33. doi: 10.1002/jcp.24013. J Cell Physiol. 2012. PMID: 22105830 Free PMC article.
-
DHA derivatives of fish oil as dietary supplements: a nutrition-based drug discovery approach for therapies to prevent metabolic cardiotoxicity.Expert Opin Drug Discov. 2012 Aug;7(8):711-21. doi: 10.1517/17460441.2012.694862. Epub 2012 Jun 24. Expert Opin Drug Discov. 2012. PMID: 22724444 Free PMC article. Review.
References
-
- Falanga V. (2005) Lancet 366, 1736–1743 - PubMed
-
- Javazon E. H., Keswani S. G., Badillo A. T., Crombleholme T. M., Zoltick P. W., Radu A. P., Kozin E. D., Beggs K., Malik A. A., Flake A. W. (2007) Wound Repair Regen. 15, 350–359 - PubMed
-
- Montori V. M., Farmer A., Wollan P. C., Dinneen S. F. (2000) Diabetes Care 23, 1407–1415 - PubMed
-
- Shingel K. I., Faure M. P., Azoulay L., Roberge C., Deckelbaum R. J. (2008) J. Tissue Eng. Regen. Med. 2, 383–393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

