Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation
- PMID: 21112563
- PMCID: PMC3019762
- DOI: 10.1016/j.stem.2010.11.013
Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation
Abstract
The Polycomb group transcriptional repressor Bmi-1 is often upregulated in prostate cancer, but its functional roles in prostate stem cell maintenance and prostate cancer are unclear. Loss- and gain-of-function analysis in a prostate sphere assay indicates that Bmi-1 expression is required for self-renewal activity and maintenance of p63(+) stem cells. Loss of Bmi-1 blocks the self-renewal activity induced by heightened β-catenin signaling, suggesting that Bmi-1 is required for full activity of another self-renewal pathway. In vivo, Bmi-1 expression is necessary for normal prostate tubule regeneration. Altered self-renewal and proliferation through Bmi-1 modulation diminishes the susceptibility of prostate cells to transformation. In an in vivo prostate regeneration system, Bmi-1 inhibition protects prostate cells from FGF10-driven hyperplasia and slows the growth of aggressive Pten-deletion-induced prostate cancer. We conclude that Bmi-1 is a crucial regulator of self-renewal in adult prostate cells and plays important roles in prostate cancer initiation and progression.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures

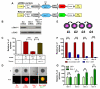

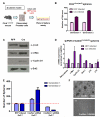

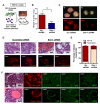

Comment in
-
An old player on a new playground: bmi-1 as a regulator of prostate stem cells.Cell Stem Cell. 2010 Dec 3;7(6):639-40. doi: 10.1016/j.stem.2010.11.019. Cell Stem Cell. 2010. PMID: 21112554 No abstract available.
-
Bmi-1, stem cells and prostate carcinogenesis.Asian J Androl. 2011 May;13(3):353-4. doi: 10.1038/aja.2011.7. Epub 2011 Feb 21. Asian J Androl. 2011. PMID: 21336300 Free PMC article. No abstract available.
Similar articles
-
Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo.Dev Biol. 2009 Apr 15;328(2):257-72. doi: 10.1016/j.ydbio.2009.01.020. Epub 2009 Jan 27. Dev Biol. 2009. PMID: 19389366 Free PMC article.
-
Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation.Nature. 2003 Oct 30;425(6961):962-7. doi: 10.1038/nature02060. Epub 2003 Oct 22. Nature. 2003. PMID: 14574365 Free PMC article.
-
Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer.Cell Cycle. 2006 Aug;5(16):1886-901. doi: 10.4161/cc.5.16.3222. Epub 2006 Aug 15. Cell Cycle. 2006. PMID: 16963837
-
Stem cell divisions controlled by the proto-oncogene BMI-1.J Stem Cells. 2009;4(3):141-6. J Stem Cells. 2009. PMID: 20232599 Review.
-
[Regulatory effects of Bmi-1 gene on self-renewal of hematopoietic stem cells--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006 Apr;14(2):413-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006. PMID: 16638228 Review. Chinese.
Cited by
-
Prostate tumorigenesis induced by PTEN deletion involves estrogen receptor β repression.Cell Rep. 2015 Mar 31;10(12):1982-91. doi: 10.1016/j.celrep.2015.02.063. Epub 2015 Mar 26. Cell Rep. 2015. PMID: 25818291 Free PMC article.
-
Akt-mediated phosphorylation of Bmi1 modulates its oncogenic potential, E3 ligase activity, and DNA damage repair activity in mouse prostate cancer.J Clin Invest. 2012 May;122(5):1920-32. doi: 10.1172/JCI57477. Epub 2012 Apr 16. J Clin Invest. 2012. PMID: 22505453 Free PMC article.
-
Jak2-Stat5a/b Signaling Induces Epithelial-to-Mesenchymal Transition and Stem-Like Cell Properties in Prostate Cancer.Am J Pathol. 2015 Sep;185(9):2505-22. doi: 10.1016/j.ajpath.2015.04.026. Am J Pathol. 2015. PMID: 26362718 Free PMC article.
-
Dysregulation of Bmi1 promotes malignant transformation of hepatic progenitor cells.Oncogenesis. 2016 Feb 29;5(2):e203. doi: 10.1038/oncsis.2016.6. Oncogenesis. 2016. PMID: 26926789 Free PMC article.
-
O-GlcNAcylation modulates Bmi-1 protein stability and potential oncogenic function in prostate cancer.Oncogene. 2017 Nov 9;36(45):6293-6305. doi: 10.1038/onc.2017.223. Epub 2017 Jul 17. Oncogene. 2017. PMID: 28714959
References
-
- Asselin-Labat ML, Vaillant F, Shackleton M, Bouras T, Lindeman GJ, Visvader JE. Delineating the epithelial hierarchy in the mouse mammary gland. Cold Spring Harb Symp Quant Biol. 2008;73:469–478. - PubMed
-
- Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–697. - PubMed
-
- Carson CC., 3rd Carcinoma of the prostate: overview of the most common malignancy in men. N C Med J. 2006;67:122–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

