Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains
- PMID: 21111236
- PMCID: PMC3058345
- DOI: 10.1016/j.cell.2010.10.003
Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains
Abstract
The functional consequences of signaling receptor endocytosis are determined by the endosomal sorting of receptors between degradation and recycling pathways. How receptors recycle efficiently, in a sequence-dependent manner that is distinct from bulk membrane recycling, is not known. Here, in live cells, we visualize the sorting of a prototypical sequence-dependent recycling receptor, the beta-2 adrenergic receptor, from bulk recycling proteins and the degrading delta-opioid receptor. Our results reveal a remarkable diversity in recycling routes at the level of individual endosomes, and indicate that sequence-dependent recycling is an active process mediated by distinct endosomal subdomains distinct from those mediating bulk recycling. We identify a specialized subset of tubular microdomains on endosomes, stabilized by a highly localized but dynamic actin machinery, that mediate this sorting, and provide evidence that these actin-stabilized domains provide the physical basis for a two-step kinetic and affinity-based model for protein sorting into the sequence-dependent recycling pathway.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
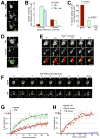
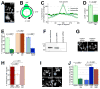
Comment in
-
Endocytosis: Sorting the recycling.Nat Rev Mol Cell Biol. 2011 Jan;12(1):3. doi: 10.1038/nrm3038. Nat Rev Mol Cell Biol. 2011. PMID: 21179055 No abstract available.
Similar articles
-
Src regulates sequence-dependent beta-2 adrenergic receptor recycling via cortactin phosphorylation.Traffic. 2014 Nov;15(11):1195-205. doi: 10.1111/tra.12202. Epub 2014 Sep 8. Traffic. 2014. PMID: 25077552 Free PMC article.
-
Distinct G protein-coupled receptor recycling pathways allow spatial control of downstream G protein signaling.J Cell Biol. 2016 Sep 26;214(7):797-806. doi: 10.1083/jcb.201512068. Epub 2016 Sep 19. J Cell Biol. 2016. PMID: 27646272 Free PMC article.
-
Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb.Traffic. 2008 Nov;9(11):1958-71. doi: 10.1111/j.1600-0854.2008.00813.x. Epub 2008 Aug 9. Traffic. 2008. PMID: 18785920 Free PMC article.
-
Endosome biogenesis is controlled by ER and the cytoskeleton at tripartite junctions.Curr Opin Cell Biol. 2023 Feb;80:102155. doi: 10.1016/j.ceb.2023.102155. Epub 2023 Feb 26. Curr Opin Cell Biol. 2023. PMID: 36848759 Review.
-
Endosomal microdomains: Formation and function.Curr Opin Cell Biol. 2020 Aug;65:86-95. doi: 10.1016/j.ceb.2020.02.018. Epub 2020 Apr 1. Curr Opin Cell Biol. 2020. PMID: 32247230 Free PMC article. Review.
Cited by
-
Postendocytic Sorting of Adrenergic and Opioid Receptors: New Mechanisms and Functions.Prog Mol Biol Transl Sci. 2015;132:189-206. doi: 10.1016/bs.pmbts.2015.03.005. Epub 2015 Apr 11. Prog Mol Biol Transl Sci. 2015. PMID: 26055059 Free PMC article. Review.
-
Towards a minimal stochastic model for a large class of diffusion-reactions on biological membranes.J Chem Phys. 2012 Aug 28;137(8):084103. doi: 10.1063/1.4746692. J Chem Phys. 2012. PMID: 22938214 Free PMC article.
-
Mechanisms of AMPA Receptor Endosomal Sorting.Front Mol Neurosci. 2018 Dec 5;11:440. doi: 10.3389/fnmol.2018.00440. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30568574 Free PMC article. Review.
-
Coronin 1C promotes triple-negative breast cancer invasiveness through regulation of MT1-MMP traffic and invadopodia function.Oncogene. 2018 Dec;37(50):6425-6441. doi: 10.1038/s41388-018-0422-x. Epub 2018 Jul 31. Oncogene. 2018. PMID: 30065298
-
Retromer: a master conductor of endosome sorting.Cold Spring Harb Perspect Biol. 2014 Feb 1;6(2):a016774. doi: 10.1101/cshperspect.a016774. Cold Spring Harb Perspect Biol. 2014. PMID: 24492709 Free PMC article. Review.
References
-
- Brown MS, Anderson RG, Goldstein JL. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983;32:663–7. - PubMed
-
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β 2-adrenergic receptor. Nature. 1999;401:286–290. - PubMed
-
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

